- Record: found
- Abstract: found
- Article: found
Ovarian cancer: Current status and strategies for improving therapeutic outcomes

Read this article at
Abstract
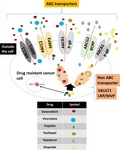
Of all the gynecologic tumors, ovarian cancer (OC) is known to be the deadliest. Advanced‐stages of OC are linked with high morbidity and low survival rates despite the immense amount of research in the field. Shortage of promising screening tools for early‐stage detection is one of the major challenges linked with the poor survival rate for patients with OC. In OC, therapeutic management is used with multidisciplinary approaches that includes debulking surgery, chemotherapy, and (rarely) radiotherapy. Recently, there is an increasing interest in using immunomodulation for treating OC. Relapse rates are high in this malignancy and averages around every 2‐years. Further treatments after the relapse are more intense, increasing the toxicity, resistance to chemotherapy drugs, and financial burden to patients with poor quality‐of‐life. A procedure that has been studied to help reduce the morbidity rate involves pre‐sensitizing cancer cells with standard therapy in order to produce optimal results with minimum dosage. Utilizing such an approach, platinum‐based agents are effective due to their increased response to platinum‐based chemotherapy in relapsed cases. These chemo‐drugs also help address the issue of drug resistance. After conducting an extensive search with available literature and the resources for clinical trials, information is precisely documented on current research, biomarkers, options for treatment and clinical trials. Several schemes for enhancing the therapeutic responses for OC are discussed systematically in this review with an attempt in summarizing the recent developments in this exciting field of translational/clinical research.
Abstract
This review article provides an in‐depth information on current treatment strategies and available clinical trials for ovarian cancer. Systematic review of strategies for enhancing the therapeutic response in ovarian carcinoma summarizing the recent developments in translational and clinical research.
Related collections
Most cited references88
- Record: found
- Abstract: found
- Article: not found
Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma.
- Record: found
- Abstract: found
- Article: not found
Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
- Record: found
- Abstract: found
- Article: not found