- Record: found
- Abstract: found
- Article: found
Outcomes and drivers of inappropriate dosing of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation: a systematic review and meta-analysis
Read this article at
Abstract
Objective
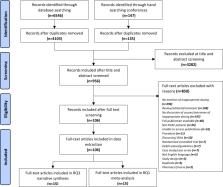
There has been limited systematic evaluation of outcomes and drivers of inappropriate non-vitamin K antagonist oral anticoagulants (NOACs) dosing among patients with atrial fibrillation (AF). This review identified and systematically evaluated literature on clinical and economic outcomes of inappropriate NOAC dosing and associated patient characteristics.
Methods
MEDLINE, Embase, Cochrane Library, International Pharmaceutical Abstracts, Econlit, PubMed and NHS EEDs databases were searched for English language observational studies from all geographies published between 2008 and 2020, examining outcomes of, or factors associated with, inappropriate NOAC dosing in adult patients with AF.
Results
One hundred and six studies were included in the analysis. Meta-analysis showed that compared with recommended NOAC dosing, off-label underdosing was associated with a null effect on stroke outcomes (ischaemic stroke and stroke/transient ischaemic attack (TIA), stroke/systemic embolism (SE) and stroke/SE/TIA). Meta-analysis of 15 studies examining clinical outcomes of inappropriate NOAC dosing found a null effect of underdosing on bleeding outcomes (major bleeding HR=1.04, 95% CI 0.90 to 1.19; p=0.625) but an increased risk of all-cause mortality (HR=1.28, 95% CI 1.10 to 1.49; p=0.006). Overdosing was associated with an increased risk of major bleeding (HR=1.41, 95% CI 1.07 to 1.85; p=0.013). No studies were found examining economic outcomes of inappropriate NOAC dosing. Narrative synthesis of 12 studies examining drivers of inappropriate NOAC dosing found that increased age, history of minor bleeds, hypertension, congestive heart failure and low creatine clearance (CrCl) were associated with an increased risk of underdosing. There was insufficient evidence to assess drivers of overdosing.
Conclusions
Our analysis suggests that off-label underdosing of NOACs does not reduce bleeding outcomes. Patients prescribed off-label NOAC doses are at an increased risk of all-cause mortality. These data underscore the importance of prescriber adherence to NOAC dosing guidelines to achieve optimal clinical outcomes for patients with AF.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Rivaroxaban versus warfarin in nonvalvular atrial fibrillation.
- Record: found
- Abstract: found
- Article: not found