- Record: found
- Abstract: found
- Article: found
The FGFR1 inhibitor PD173074 induces mesenchymal–epithelial transition through the transcription factor AP-1
Read this article at
Abstract
Background:
Epithelial–mesenchymal transition (EMT) is a crucial process in cancer progression that provides cancer cells with the ability to escape from the primary focus, invade stromal tissues and migrate to distant regions. Cell lines that lack E-cadherin show increased tumorigenesis and metastasis, and the expression levels of E-cadherin and Snail correlate inversely with the prognosis of patients suffering from breast cancer or oral squamous cell carcinoma (OSCC). Moreover, recent studies have shown that most EMT cases are regulated by soluble growth factors or cytokines. Among these factors, fibroblast growth factors (FGFs) execute diverse functions by binding to and activating members of the FGF receptor (FGFR) family, including FGFR1–4. Fibroblast growth factor receptor 1 is an oncoprotein that is involved in tumorigenesis, and PD173074 is known to be a selective inhibitor of FGFR1. However, the roles of FGFR1 and FGFR1 inhibitors have not yet been examined in detail.
Methods:
Here, we investigated the expression of FGFR1 in head and neck squamous cell carcinoma (HNSCC) and the role of the FGFR1 inhibitor PD173074 in carcinogenesis and the EMT process.
Results:
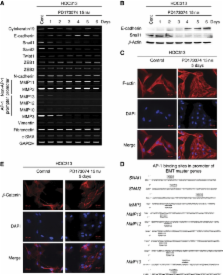
Fibroblast growth factor receptor 1 was highly expressed in 54% of HNSCC cases and was significantly correlated with malignant behaviours. Nuclear FGFR1 expression was also observed and correlated well with histological differentiation, the pattern of invasion and abundant nuclear polymorphism. Fibroblast growth factor receptor 1 was also overexpressed in EMT cell lines compared with non-EMT cell lines. Furthermore, treatment of HOC313 cells with PD173074 suppressed cellular proliferation and invasion and reduced ERK1/2 and p38 activation. These cells also demonstrated morphological changes, transforming from spindle- to cobble stone-like in shape. In addition, the expression levels of certain matrix metalloproteinases (MMPs), whose genes contain activator protein-1 (AP-1) promoter sites, as well as Snail1 and Snail2 were reduced following PD173074 treatment.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Regulation of cadherin-mediated adhesion in morphogenesis.
- Record: found
- Abstract: not found
- Article: not found
Cell adhesion and signalling by cadherins and Ig-CAMs in cancer.
- Record: found
- Abstract: found
- Article: not found
FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.