- Record: found
- Abstract: found
- Article: found
Resurrecting biodiversity: advanced assisted reproductive technologies and biobanking

Abstract
Biodiversity is defined as the presence of a variety of living organisms on the Earth that is essential for human survival. However, anthropogenic activities are causing the sixth mass extinction, threatening even our own species. For many animals, dwindling numbers are becoming fragmented populations with low genetic diversity, threatening long-term species viability. With extinction rates 1000–10,000 times greater than natural, ex situ and in situ conservation programmes need additional support to save species. The indefinite storage of cryopreserved (−196°C) viable cells and tissues (cryobanking), followed by assisted or advanced assisted reproductive technology (ART: utilisation of oocytes and spermatozoa to generate offspring; aART: utilisation of somatic cell genetic material to generate offspring), may be the only hope for species’ long-term survival. As such, cryobanking should be considered a necessity for all future conservation strategies. Following cryopreservation, ART/aART can be used to reinstate lost genetics back into a population, resurrecting biodiversity. However, for this to be successful, species-specific protocol optimisation and increased knowledge of basic biology for many taxa are required. Current ART/aART is primarily focused on mammalian taxa; however, this needs to be extended to all, including to some of the most endangered species: amphibians. Gamete, reproductive tissue and somatic cell cryobanking can fill the gap between losing genetic diversity today and future technological developments. This review explores species prioritisation for cryobanking and the successes and challenges of cryopreservation and multiple ARTs/aARTs. We here discuss the value of cryobanking before more species are lost and the potential of advanced reproductive technologies not only to halt but also to reverse biodiversity loss.
Lay summary
The world is undergoing its sixth mass extinction; however, unlike previous events, the latest is caused by human activities and is resulting in the largest loss of biodiversity (all living things on Earth) for 65 million years. With an extinction rate 1000–10,000-fold greater than natural, this catastrophic decline in biodiversity is threatening our own survival. As the number of individuals within a species declines, genetic diversity reduces, threatening their long-term existence. In this review, the authors summarise approaches to indefinitely preserve living cells and tissues at low temperatures (cryobanking) and the technologies required to resurrect biodiversity. In the future when appropriate techniques become available, these living samples can be thawed and used to reinstate genetic diversity and produce live young ones of endangered species, enabling their long-term survival. The successes and challenges of genome resource cryopreservation are discussed to enable a move towards a future of stable biodiversity.
Related collections
Most cited references307
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
- Record: found
- Abstract: found
- Article: not found
Genome editing. The new frontier of genome engineering with CRISPR-Cas9.
- Record: found
- Abstract: found
- Article: found
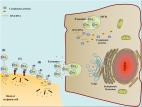
Exosomes: biogenesis, biologic function and clinical potential
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.

 This work is licensed under a
This work is licensed under a