- Record: found
- Abstract: found
- Article: found
TLR4 promotes microglial pyroptosis via lncRNA-F630028O10Rik by activating PI3K/AKT pathway after spinal cord injury
Read this article at
Abstract
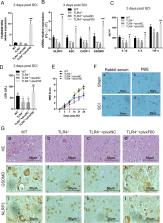
Neuroinflammation plays a crucial role in the secondary phase of spinal cord injury (SCI), and is initiated following the activation of toll-like receptor 4 (TLR4). However, the downstream mechanism remains unknown. Pyroptosis is a form of inflammatory programmed cell death, which is closely involved in neuroinflammation, and it can be regulated by TLR4 according to a recent research. In addition, several studies have shown that long non-coding RNAs (lncRNAs) based mechanisms were related to signal transduction downstream of TLR4 in the regulation of inflammation. Thus, in this study, we want to determine whether TLR4 can regulate pyroptosis after SCI via lncRNAs. Our results showed that TLR4 was activated following SCI and promoted the expression of lncRNA-F630028O10Rik. This lncRNA functioned as a ceRNA for miR-1231-5p/Col1a1 axis and enhanced microglial pyroptosis after SCI by activating the PI3K/AKT pathway. Furthermore, we determined STAT1 was the upstream transcriptional factor of IncRNA-F630028O10Rik and was induced by the damage-responsive TLR4/MyD88 signal. Our findings provide new insights and a novel therapeutic strategy for treating SCI.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Inflammasomes in the CNS.
- Record: found
- Abstract: found
- Article: not found