- Record: found
- Abstract: found
- Article: found
The prognostic value of fibrinogen to albumin ratio in malignant tumor patients: A meta-analysis
Read this article at
Abstract
Background
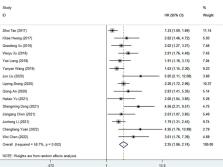
Recent studies have shown that the fibrinogen to albumin ratio (FAR) is closely related to the prognosis of various cancers. The aim of this systematic review and meta-analysis was to investigate the prognostic value of FAR in malignancies based on the available evidence.
Method
To systematically search the Cochrane Library, Embase, PubMed, Google Scholar, Baidu scholars, CNKI and VIP databases for relevant studies published before April 1, 2022, and to evaluate the fibrinogen-to-albumin ratio (FAR) and survival of patients with malignant tumors through a meta-analysis relationship between the results. Results. This meta-analysis included 19 eligible studies involving 5926 cancer patients. We found that high FAR was associated with poor overall survival (HR=2.25, 95%CI 1.86-2.74, p<0.001), recurrence-free survival (HR=2.29, 95%CI 1.91-2.76, P<0.001), progression-free survival (HR: 2.10, 95%CI 1.58-2.79, p<0.001), disease-free survival (HR=1.52, 95%CI 1.17-1.96, p=0.001), and time to recurrence (HR: 1.555, 95%CI 1.031-2.346, P=0.035) was significantly correlated.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
- Record: found
- Abstract: not found
- Article: not found