- Record: found
- Abstract: found
- Article: found
Prion protein with a mutant N-terminal octarepeat region undergoes cobalamin-dependent assembly into high–molecular weight complexes
Read this article at
Abstract
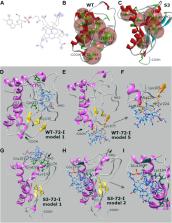
The cellular prion protein (PrP C) has a C-terminal globular domain and a disordered N-terminal region encompassing five octarepeats (ORs). Encounters between Cu(II) ions and four OR sites produce interchangeable binding geometries; however, the significance of Cu(II) binding to ORs in different combinations is unclear. To understand the impact of specific binding geometries, OR variants were designed that interact with multiple or single Cu(II) ions in specific locked coordinations. Unexpectedly, we found that one mutant produced detergent-insoluble, protease-resistant species in cells in the absence of exposure to the infectious prion protein isoform, scrapie-associated prion protein (PrP Sc). Formation of these assemblies, visible as puncta, was reversible and dependent upon medium formulation. Cobalamin (Cbl), a dietary cofactor containing a corrin ring that coordinates a Co 3+ ion, was identified as a key medium component, and its effect was validated by reconstitution experiments. Although we failed to find evidence that Cbl interacts with Cu-binding OR regions, we instead noted interactions of Cbl with the PrP C C-terminal domain. We found that some interactions occurred at a binding site of planar tetrapyrrole compounds on the isolated globular domain, but others did not, and N-terminal sequences additionally had a marked effect on their presence and position. Our studies define a conditional effect of Cbl wherein a mutant OR region can act in cis to destabilize a globular domain with a wild type sequence. The unexpected intersection between the properties of PrP Sc's disordered region, Cbl, and conformational remodeling events may have implications for understanding sporadic prion disease that does not involve exposure to PrP Sc.
Related collections
Most cited references117
- Record: found
- Abstract: found
- Article: not found
UCSF Chimera--a visualization system for exploratory research and analysis.
- Record: found
- Abstract: found
- Article: not found
AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading.
- Record: found
- Abstract: found
- Article: not found