- Record: found
- Abstract: found
- Article: found
Bergenin Exerts Hepatoprotective Effects by Inhibiting the Release of Inflammatory Factors, Apoptosis and Autophagy via the PPAR-γ Pathway
Abstract
Objective
Hepatic ischemia reperfusion (IR) limits the development of liver transplantation technology. The aim of this study was to explore the protective effects of Bergenin on hepatic IR, particularly the elimination of reactive oxygen species (ROS) and activation of the peroxisome proliferators activated receptor γ (PPAR-γ) pathway.
Methods
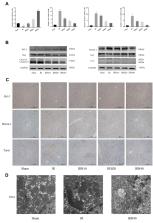
Initial experiments were performed to confirm the non-toxicity of Bergenin. Mice were randomly divided into sham, IR, and IR + Bergenin (10, 20 and 40 mg/kg) groups, and serum and tissue samples were obtained at 2, 8 and 24 h for detection of liver enzymes (ALT and AST), inflammatory factors (TNF-α, IL-6 and IL-1β), ROS, cell death markers (Bcl-2, Bax, Beclin-1 and LC3) and related important pathways (PPAR-γ, P38 MAPK, NF-κB p65 and JAK2/STAT1).
Results
Bergenin reduced the release of ROS, down-regulated inflammatory factors, and inhibited apoptosis and autophagy. Additionally, expression of PPAR-γ-related genes was increased and phosphorylation of P38 MAPK, NF-κB p65 and JAK2/STAT1-related proteins was decreased in Bergenin pre-treatment groups in a dose-dependent manner.
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo.

- Record: found
- Abstract: found
- Article: found
Astaxanthin Pretreatment Attenuates Hepatic Ischemia Reperfusion-Induced Apoptosis and Autophagy via the ROS/MAPK Pathway in Mice
- Record: found
- Abstract: found
- Article: not found