- Record: found
- Abstract: found
- Article: not found
A pathway linking oxidative stress and the Ran GTPase system in progeria
Read this article at
Abstract
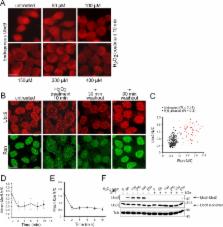
In progeria, a mutant form of lamin A constitutively tethered to the inner nuclear membrane causes disruption of the Ran GTPase system by inducing ROS. However, ROS are also induced by disruption of the Ran system. The data suggest that the nuclear lamina and Ran GTPase system are part of a pathway that contains positive and negative feedback loops.
Abstract
Maintaining the Ran GTPase at a proper concentration in the nucleus is important for nucleocytoplasmic transport. Previously we found that nuclear levels of Ran are reduced in cells from patients with Hutchinson–Gilford progeria syndrome (HGPS), a disease caused by constitutive attachment of a mutant form of lamin A (termed progerin) to the nuclear membrane. Here we explore the relationship between progerin, the Ran GTPase, and oxidative stress. Stable attachment of progerin to the nuclear membrane disrupts the Ran gradient and results in cytoplasmic localization of Ubc9, a Ran-dependent import cargo. Ran and Ubc9 disruption can be induced reversibly with H 2O 2. CHO cells preadapted to oxidative stress resist the effects of progerin on Ran and Ubc9. Given that HGPS-patient fibroblasts display elevated ROS, these data suggest that progerin inhibits nuclear transport via oxidative stress. A drug that inhibits pre–lamin A cleavage mimics the effects of progerin by disrupting the Ran gradient, but the effects on Ran are observed before a substantial ROS increase. Moreover, reducing the nuclear concentration of Ran is sufficient to induce ROS irrespective of progerin. We speculate that oxidative stress caused by progerin may occur upstream or downstream of Ran, depending on the cell type and physiological setting.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion.
- Record: found
- Abstract: found
- Article: not found
Nuclear lamins and laminopathies.
- Record: found
- Abstract: found
- Article: not found
