- Record: found
- Abstract: found
- Article: found
Self-Organizing Properties of Mouse Pluripotent Cells Initiate Morphogenesis upon Implantation
Read this article at
Summary
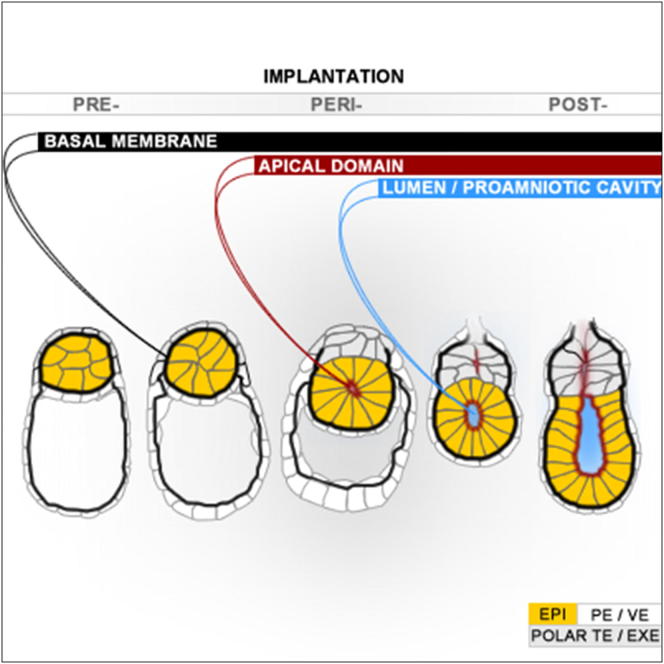
Transformation of pluripotent epiblast cells into a cup-shaped epithelium as the mouse blastocyst implants is a poorly understood and yet key developmental step. Studies of morphogenesis in embryoid bodies led to the current belief that it is programmed cell death that shapes the epiblast. However, by following embryos developing in vivo and in vitro, we demonstrate that not cell death but a previously unknown morphogenetic event transforms the amorphous epiblast into a rosette of polarized cells. This transformation requires basal membrane-stimulated integrin signaling that coordinates polarization of epiblast cells and their apical constriction, a prerequisite for lumenogenesis. We show that basal membrane function can be substituted in vitro by extracellular matrix (ECM) proteins and that ES cells can be induced to form similar polarized rosettes that initiate lumenogenesis. Together, these findings lead to a completely revised model for peri-implantation morphogenesis in which ECM triggers the self-organization of the embryo’s stem cells.
Graphical Abstract
Highlights
-
•
Apoptosis is not essential for the peri-implantation morphogenesis, as believed
-
•
Basal membrane proteins create a niche for EPI and drive morphogenesis in ES cells
-
•
Polarization and apical constriction reorganize the EPI into a rosette
-
•
The proamniotic cavity is formed through hollowing mechanism
Abstract
The first morphogenetic event by pluripotent stem cells of mouse blastocyst entails epiblast cell polarization and arrangement into a rosette pattern, which parts at its center, leading to cavity formation.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells.
- Record: found
- Abstract: not found
- Article: not found
Establishment in culture of pluripotential cells from mouse embryos.
- Record: found
- Abstract: found
- Article: not found