- Record: found
- Abstract: found
- Article: found
Seroconversion rate after COVID-19 vaccination in patients with solid cancer: A systematic review and meta-analysis

Read this article at
ABSTRACT
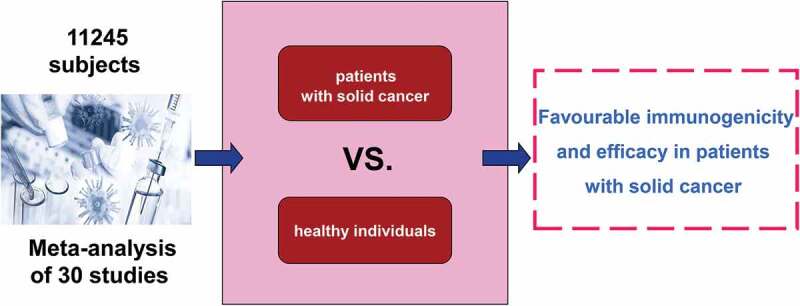
Patients with solid cancer have an increased risk of severe coronavirus disease 2019 (COVID-19) and associated mortality than the general population. This meta-analysis aimed to investigate the currently available evidence about the efficacy of COVID-19 vaccines in patients with solid cancer. We included prospective studies comparing the immunogenicity and efficacy of COVID-19 vaccines between patients with solid cancer and healthy individuals. Relative risks of seroconversion after the first and second dose of a COVID-19 vaccine were separately pooled with the use of random effects meta-analysis. Thirty studies with 11,245 subjects met the inclusion criteria. After first vaccine dose, the pooled RR of seroconversion in patients with solid cancer vs healthy individuals was 0.54 (95% CI 0.38–0.78, I 2 = 94%). After a second dose, the pooled RR of seroconversion in patients with solid cancer vs healthy controls was 0.87 (0.86–0.88, I 2 = 87%). Our review suggests that, compared with healthy individuals, COVID-19 vaccines show favorable immunogenicity and efficacy in patients with solid cancer. A second dose is associated with significantly improved seroconversion, although it is slightly lower in patients with solid cancer compared with healthy individuals.
Graphical abstract
Related collections
Most cited references102

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
- Record: found
- Abstract: not found
- Article: not found
RoB 2: a revised tool for assessing risk of bias in randomised trials
- Record: found
- Abstract: found
- Article: not found