- Record: found
- Abstract: found
- Article: found
Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy
Abstract
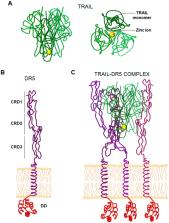
(TNF)-related apoptosis-inducing ligand (TRAIL) is able to activate the extrinsic apoptotic pathway upon binding to DR4/TRAIL-R1 and/or DR5/TRAIL-R2 receptors. Structural data indicate that TRAIL functions as a trimer that can engage three receptor molecules simultaneously, resulting in receptor trimerization and leading to conformational changes in TRAIL receptors. However, receptor conformational changes induced by the binding of TRAIL depend on the molecular form of this death ligand, and not always properly trigger the apoptotic cascade. In fact, TRAIL exhibits a much stronger pro-apoptotic activity when is found as a transmembrane protein than when it occurs as a soluble form and this enhanced biological activity is directly linked to its ability to cluster TRAIL receptors in supra-molecular structures. In this regard, cells involved in tumor immunosurveillance, such as activated human T cells, secrete endogenous TRAIL as a transmembrane protein associated with lipid microvesicles called exosomes upon T-cell reactivation. Consequently, it seems clear that a proper oligomerization of TRAIL receptors, which leads to a strong apoptotic signaling, is crucial for inducing apoptosis in cancer cells upon TRAIL treatment. In this review, the current knowledge of oligomerization status of TRAIL receptors is discussed as well as the implications for cancer treatment when using TRAIL-based therapies.
Related collections
Most cited references145
- Record: found
- Abstract: found
- Article: not found
FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis.
- Record: found
- Abstract: not found
- Article: not found
The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation
- Record: found
- Abstract: found
- Article: found