- Record: found
- Abstract: found
- Article: found
Pharmacokinetic serum concentrations of VRC01 correlate with prevention of HIV-1 acquisition
Read this article at
Summary
Background
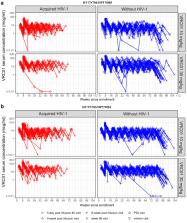
The phase 2b proof-of-concept Antibody Mediated Prevention (AMP) trials showed that VRC01, an anti-HIV-1 broadly neutralising antibody (bnAb), prevented acquisition of HIV-1 sensitive to VRC01. To inform future study design and dosing regimen selection of candidate bnAbs, we investigated the association of VRC01 serum concentration with HIV-1 acquisition using AMP trial data.
Methods
The case–control sample included 107 VRC01 recipients who acquired HIV-1 and 82 VRC01 recipients who remained without HIV-1 during the study. We measured VRC01 serum concentrations with a qualified pharmacokinetic (PK) Binding Antibody Multiplex Assay. We employed nonlinear mixed effects PK modelling to estimate daily-grid VRC01 concentrations. Cox regression models were used to assess the association of VRC01 concentration at exposure and baseline body weight, with the hazard of HIV-1 acquisition and prevention efficacy as a function of VRC01 concentration. We also compared fixed dosing vs. body weight-based dosing via simulations.
Findings
Estimated VRC01 concentrations in VRC01 recipients without HIV-1 were higher than those in VRC01 recipients who acquired HIV-1. Body weight was inversely associated with HIV-1 acquisition among both placebo and VRC01 recipients but did not modify the prevention efficacy of VRC01. VRC01 concentration was inversely correlated with HIV-1 acquisition, and positively correlated with prevention efficacy of VRC01. Simulation studies suggest that fixed dosing may be comparable to weight-based dosing in overall predicted prevention efficacy.
Interpretation
These findings suggest that bnAb serum concentration may be a useful marker for dosing regimen selection, and operationally efficient fixed dosing regimens could be considered for future trials of HIV-1 bnAbs.
Funding
Was provided by the doi 10.13039/100000002, National Institutes of Health; , doi 10.13039/100000060, National Institute of Allergy and Infectious Diseases; (NIAID) (UM1 AI068614, to the HIV Vaccine Trials Network [HVTN]; UM1 AI068635, to the HVTN Statistical Data and Management Center [SDMC], Fred Hutchinson Cancer Center [FHCC]; 2R37 054165 to the FHCC; UM1 AI068618, to HVTN Laboratory Center, FHCC; UM1 AI068619, to the HPTN Leadership and Operations Center; UM1 AI068613, to the HIV Prevention Trials Network [HPTN] Laboratory Center; UM1 AI068617, to the HPTN SDMC; and P30 AI027757, to the Center for AIDS Research, doi 10.13039/100006510, Duke University; (AI P30 AI064518) and doi 10.13039/100007812, University of Washington; (P30 AI027757) Centers for AIDS Research; R37AI054165 from NIAID to the FHCC; and OPP1032144 CA-VIMC doi 10.13039/100000865, Bill & Melinda Gates Foundation; .
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection.
- Record: found
- Abstract: found
- Article: not found
Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition
- Record: found
- Abstract: found
- Article: not found