- Record: found
- Abstract: found
- Article: found
On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy
Read this article at
Abstract
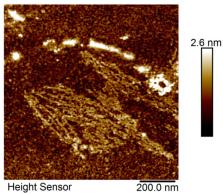
Oligomeric intermediates on the pathway of amyloid fibrillation are suspected as the main cytotoxins responsible for amyloid-related pathogenicity. As they appear to be a part of the lag phase of amyloid fibrillation when analyzed using standard methods such as Thioflavin T (ThT) fluorescence, a more sensitive method is needed for their detection. Here we apply Fourier transform infrared spectroscopy (FTIR) in attenuated total reflectance (ATR) mode for fast and cheap analysis of destabilized hen-egg-white lysozyme solution and detection of oligomer intermediates of amyloid fibrillation. Standard methods of protein aggregation analysis— Thioflavin T (ThT) fluorescence, atomic force microscopy (AFM), and 8-anilinonaphthalene-1-sulphonic acid (ANS) fluorescence were applied and compared to FTIR spectroscopy data. Results show the great potential of FTIR for both, qualitative and quantitative monitoring of oligomer formation based on the secondary structure changes. While oligomer intermediates do not induce significant changes in ThT fluorescence, their secondary structure changes were very prominent. Normalization of specific Amide I region peak intensities by using Amide II peak intensity as an internal standard provides an opportunity to use FTIR spectroscopy for both qualitative and quantitative analysis of biological samples and detection of potentially toxic oligomers, as well as for screening of efficiency of fibrillation procedures.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Infrared spectroscopy of proteins.
- Record: found
- Abstract: found
- Article: not found
The amyloid state and its association with protein misfolding diseases.
- Record: found
- Abstract: found
- Article: not found