- Record: found
- Abstract: found
- Article: found
Endothelial type I interferon response and brain diseases: identifying STING as a therapeutic target
Read this article at
Abstract
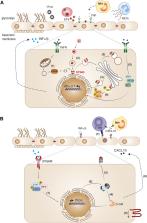
The endothelium layer lining the inner surface of blood vessels serves relevant physiological functions in all body systems, including the exchanges between blood and extravascular space. However, endothelial cells also participate in innate and adaptive immune response that contribute to the pathophysiology of inflammatory disorders. Type I Interferon (IFN) signaling is an inflammatory response triggered by a variety of pathogens, but it can also be induced by misplaced DNA in the cytosol caused by cell stress or gene mutations. Type I IFN produced by blood leukocytes or by the endothelium itself is well-known to activate the interferon receptor (IFNAR) in endothelial cells. Here, we discuss the induction of type I IFN secretion and signaling in the endothelium, specifically in the brain microvasculature where endothelial cells participate in the tight blood-brain barrier (BBB). This barrier is targeted during neuroinflammatory disorders such as infection, multiple sclerosis, Alzheimer’s disease and traumatic brain injury. We focus on type I IFN induction through the cGAS-STING activation pathway in endothelial cells in context of autoinflammatory type I interferonopathies, inflammation and infection. By comparing the pathophysiology of two separate infectious diseases—cerebral malaria induced by Plasmodium infection and COVID-19 caused by SARS-CoV-2 infection—we emphasize the relevance of type I IFN and STING-induced vasculopathy in organ dysfunction. Investigating the role of endothelial cells as active type I IFN producers and responders in disease pathogenesis could lead to new therapeutic targets. Namely, endothelial dysfunction and brain inflammation may be avoided with strategies that target excessive STING activation in endothelial cells.
Related collections
Most cited references121

- Record: found
- Abstract: found
- Article: found
Autoantibodies against type I IFNs in patients with life-threatening COVID-19
- Record: found
- Abstract: found
- Article: not found
Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris
- Record: found
- Abstract: found
- Article: not found