- Record: found
- Abstract: found
- Article: found
The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway

Read this article at
Abstract
Background
Recently, tRNA-derived fragments (tRFs) have been shown to serve important biological functions. However, the role of tRFs in gastric cancer has not been fully elucidated. This study aimed to identify the tumor suppressor role of tRF-5026a (tRF-18-79MP9P04) in gastric cancer.
Methods
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was first used to detect tRF-5026a expression levels in gastric cancer tissues and patient plasma. Next, the relationship between tRF-5026a levels and clinicopathological features in gastric cancer patients was assessed. Cell lines with varying tRF-5026a levels were assessed by measuring tRF-5026a using qRT-PCR. After transfecting cell lines with a tRF-5026a mimic or inhibitor, cell proliferation, colony formation, migration, apoptosis, and cell cycle were evaluated. The expression levels of related proteins in the PTEN/PI3K/AKT pathway were also analyzed by Western blotting. Finally, the effect of tRF-5026a on tumor growth was tested using subcutaneous tumor models in nude mice.
Results
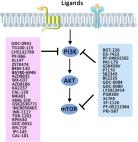
tRF-5026a was downregulated in gastric cancer patient tissues and plasma samples. tRF-5026a levels were closely related to tumor size, had a certain diagnostic value, and could be used to predict overall survival. tRF-5026a was also downregulated in gastric cancer cell lines. tRF-5026a inhibited the proliferation, migration, and cell cycle progression of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Animal experiments showed that upregulation of tRF-5026a effectively inhibited tumor growth.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: found
Targeting PI3K in cancer: mechanisms and advances in clinical trials
- Record: found
- Abstract: found
- Article: not found
Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement.

- Record: found
- Abstract: found
- Article: found