- Record: found
- Abstract: found
- Article: found
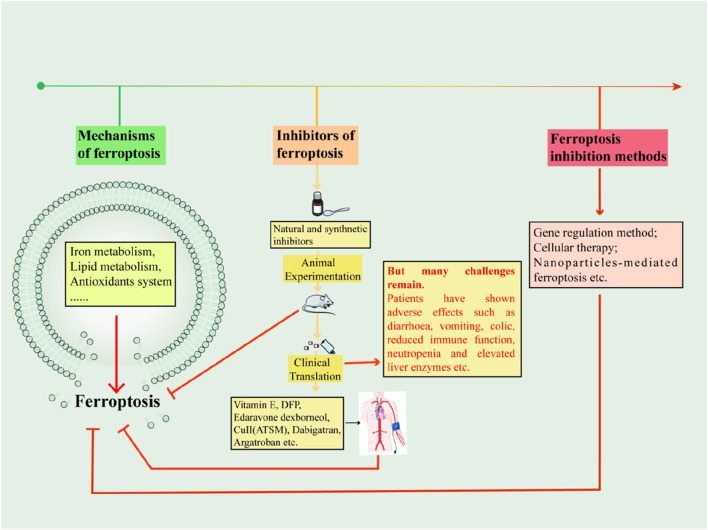
Ferroptosis inhibitors: past, present and future

Read this article at
Abstract
Ferroptosis is a non-apoptotic mode of programmed cell death characterized by iron dependence and lipid peroxidation. Since the ferroptosis was proposed, researchers have revealed the mechanisms of its formation and continue to explore effective inhibitors of ferroptosis in disease. Recent studies have shown a correlation between ferroptosis and the pathological mechanisms of neurodegenerative diseases, as well as diseases involving tissue or organ damage. Acting on ferroptosis-related targets may provide new strategies for the treatment of ferroptosis-mediated diseases. This article specifically describes the metabolic pathways of ferroptosis and summarizes the reported mechanisms of action of natural and synthetic small molecule inhibitors of ferroptosis and their efficacy in disease. The paper also describes ferroptosis treatments such as gene therapy, cell therapy, and nanotechnology, and summarises the challenges encountered in the clinical translation of ferroptosis inhibitors. Finally, the relationship between ferroptosis and other modes of cell death is discussed, hopefully paving the way for future drug design and discovery.
Graphical Abstract
Related collections
Most cited references329
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition.
- Record: found
- Abstract: found
- Article: not found