- Record: found
- Abstract: found
- Article: found
A Novel Toll-Like Receptor (TLR) Influences Compatibility between the Gastropod Biomphalaria glabrata, and the Digenean Trematode Schistosoma mansoni
Read this article at
Abstract
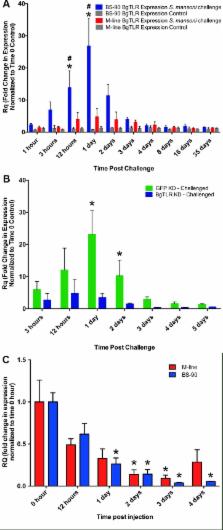
Schistosomiasis, a devastating disease caused by parasitic flatworms of the genus Schistosoma, affects over 260 million people worldwide especially in tropical and sub-tropical regions. Schistosomes must undergo their larval development within specific species of snail intermediate hosts, a trait that is shared among almost all digenean trematodes. This unique and long-standing host-parasite relationship presents an opportunity to study both the importance of conserved immunological features in novel immunological roles, as well as new immunological adaptations that have arisen to combat a very specific type of immunological challenge. While it is well supported that the snail immune response is important for protecting against schistosome infection, very few specific snail immune factors have been identified and even fewer have been functionally characterized. Here, we provide the first functional report of a snail Toll-like receptor, which we demonstrate as playing an important role in the cellular immune response of the snail Biomphalaria glabrata following challenge with Schistosoma mansoni. This TLR (BgTLR) was identified as part of a peptide screen of snail immune cell surface proteins that differed in abundance between B. glabrata snails that differ in their compatibility phenotype to challenge by S. mansoni. The S. mansoni-resistant strain of B. glabrata (BS-90) displayed higher levels of BgTLR compared to the susceptible (M-line) strain. Transcript expression of BgTLR was found to be very responsive in BS-90 snails when challenged with S. mansoni, increasing 27 fold relative to β-actin (non-immune control gene); whereas expression in susceptible M-line snails was not significantly increased. Knockdown of BgTLR in BS-90 snails via targeted siRNA oligonucleotides was confirmed using a specific anti-BgTLR antibody and resulted in a significant alteration of the resistant phenotype, yielding patent infections in 43% of the normally resistant snails, which shed S. mansoni cercariae 1-week before the susceptible controls. Our results represent the first functional characterization of a gastropod TLR, and demonstrate that BgTLR is an important snail immune receptor that is capable of influencing infection outcome following S. mansoni challenge.
Author Summary
The freshwater snail Biomphalaria glabrata is the subject of intensive research, primarily due to its biomedical importance as an intermediate host for the parasitic flatworm Schistosoma mansoni, which is a causative agent of the disease schistosomiasis–a chronic, debilitating condition that affects over 260 million people worldwide. Studies of this snail have led to the identification of many factors, some with high homology to known immune molecules in other organisms and many more that are unique with no known homology. However, research into the functional and mechanistic roles of these factors has only recently progressed. In this study, we have demonstrated the functional relevance of a Toll-like receptor (TLR) that we identified in B. glabrata. Transcriptional expression of this TLR was rapidly induced in a snail strain that is resistant to S. mansoni, in a pattern that is consistent with their phenotype. Furthermore, knockdown of the TLR resulted in 43% of resistant snails becoming infected with S. mansoni to patency. This advances our understanding of the mechanistic basis of snail-schistosome compatibility significantly, and may one day facilitate the development of tools for improving the control of schistosomiasis. It also contributes novel functionality to TLRs, one of the most evolutionarily conserved pattern-recognition receptors and cognate signalling pathways in immunity.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk.
- Record: found
- Abstract: found
- Article: not found
Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin
- Record: found
- Abstract: found
- Article: not found