- Record: found
- Abstract: found
- Article: found
Sexual dimorphism in a neuronal mechanism of spinal hyperexcitability across rodent and human models of pathological pain
Read this article at
Abstract
The prevalence and severity of many chronic pain syndromes differ across sex, and recent studies have identified differences in immune signalling within spinal nociceptive circuits as a potential mediator. Although it has been proposed that sex-specific pain mechanisms converge once they reach neurons within the superficial dorsal horn, direct investigations using rodent and human preclinical pain models have been lacking.
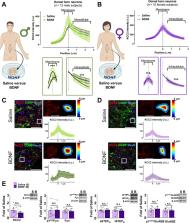
Here, we discovered that in the Freund’s adjuvant in vivo model of inflammatory pain, where both male and female rats display tactile allodynia, a pathological coupling between KCC2-dependent disinhibition and N-methyl- D-aspartate receptor (NMDAR) potentiation within superficial dorsal horn neurons was observed in male but not female rats. Unlike males, the neuroimmune mediator brain-derived neurotrophic factor (BDNF) failed to downregulate inhibitory signalling elements (KCC2 and STEP 61) and upregulate excitatory elements (pFyn, GluN2B and pGluN2B) in female rats, resulting in no effect of ex vivo brain-derived neurotrophic factor on synaptic NMDAR responses in female lamina I neurons. Importantly, this sex difference in spinal pain processing was conserved from rodents to humans. As in rodents, ex vivo spinal treatment with BDNF downregulated markers of disinhibition and upregulated markers of facilitated excitation in superficial dorsal horn neurons from male but not female human organ donors. Ovariectomy in female rats recapitulated the male pathological pain neuronal phenotype, with BDNF driving a coupling between disinhibition and NMDAR potentiation in adult lamina I neurons following the prepubescent elimination of sex hormones in females.
This discovery of sexual dimorphism in a central neuronal mechanism of chronic pain across species provides a foundational step towards a better understanding and treatment for pain in both sexes.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
Central sensitization: a generator of pain hypersensitivity by central neural plasticity.
- Record: found
- Abstract: not found
- Article: not found
Different immune cells mediate mechanical pain hypersensitivity in male and female mice.
- Record: found
- Abstract: found
- Article: not found