- Record: found
- Abstract: found
- Article: found
Superantigen-Producing Staphylococcus aureus Elicits Systemic Immune Activation in a Murine Wound Colonization Model
Read this article at
Abstract
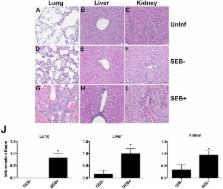
Staphylococcus aureus, the most common cause of wound infection, produces several exotoxins, including superantigens (SAgs). SAgs are the potent activators of the immune system. Given this unique property, we hypothesized that SAgs produced by S. aureus in wounds would have local, as well as systemic immunologic effects. We tested our hypothesis using a novel staphylococcal skin wound infection model in transgenic mice expressing HLA-DR3. Skin wounds were left uninfected or colonized with S. aureus strains producing SAgs or an isogenic strain not producing any SAg. Animals with wounds challenged with SAg-producing S. aureus had increased morbidity and lower serum IL-17 levels compared to those challenged with the SAg non-producing S. aureus ( p = 0.027 and p = 0.032, respectively). At Day 8 following microbial challenge, compared to mice with uninfected wounds, the proportion of Vβ8 +CD4 + T cells was increased, while the proportion of Vβ8 +CD8 + T cells was decreased only in the spleens of mice challenged with SAg-producing S. aureus ( p < 0.001). No such changes were measured in mice challenged with SAg non-producing S. aureus. Lungs, livers and kidneys from mice challenged with SAg-producing, but not SAg non-producing, S. aureus showed inflammatory changes. Overall, SAg-mediated systemic immune activation in wounds harboring S. aureus may have clinical implications.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
CD4+CD25+ TR Cells Suppress Innate Immune Pathology Through Cytokine-dependent Mechanisms
- Record: found
- Abstract: found
- Article: not found
Adaptive immune cells temper initial innate responses
- Record: found
- Abstract: found
- Article: not found