- Record: found
- Abstract: found
- Article: found
Transient Receptor Potential Ankyrin-1-expressing vagus nerve fibers mediate IL-1β induced hypothermia and reflex anti-inflammatory responses
Read this article at
Abstract
Background
Inflammation, the physiological response to infection and injury, is coordinated by the immune and nervous systems. Interleukin-1β (IL-1β) and other cytokines produced during inflammatory responses activate sensory neurons (nociceptors) to mediate the onset of pain, sickness behavior, and metabolic responses. Although nociceptors expressing Transient Receptor Potential Ankyrin-1 (TRPA1) can initiate inflammation, comparatively little is known about the role of TRPA1 nociceptors in the physiological responses to specific cytokines.
Methods
To monitor body temperature in conscious and unrestrained mice, telemetry probes were implanted into peritoneal cavity of mice. Using transgenic and tissue specific knockouts and chemogenetic techniques, we recorded temperature responses to the potent pro-inflammatory cytokine IL-1β. Using calcium imaging, whole cell patch clamping and whole nerve recordings, we investigated the role of TRPA1 during IL-1β-mediated neuronal activation. Mouse models of acute endotoxemia and sepsis were used to elucidate how specific activation, with optogenetics and chemogenetics, or ablation of TRPA1 neurons can affect the outcomes of inflammatory insults. All statistical tests were performed with GraphPad Prism 9 software and for all analyses, P ≤ 0.05 was considered statistically significant.
Results
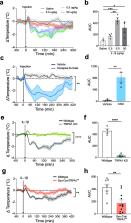
Here, we describe a previously unrecognized mechanism by which IL-1β activates afferent vagus nerve fibers to trigger hypothermia, a response which is abolished by selective silencing of neuronal TRPA1. Afferent vagus nerve TRPA1 signaling also inhibits endotoxin-stimulated cytokine storm and significantly reduces the lethality of bacterial sepsis.
Conclusion
Thus, IL-1β activates TRPA1 vagus nerve signaling in the afferent arm of a reflex anti-inflammatory response which inhibits cytokine release, induces hypothermia, and reduces the mortality of infection. This discovery establishes that TRPA1, an ion channel known previously as a pro-inflammatory detector of cold, pain, itch, and a wide variety of noxious molecules, also plays a specific anti-inflammatory role via activating reflex anti-inflammatory activity.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation.
- Record: found
- Abstract: found
- Article: not found