- Record: found
- Abstract: found
- Article: found
Immune response to the recombinant herpes zoster vaccine in people living with HIV over 50 years of age compared to non-HIV age-/gender-matched controls (SHINGR’HIV): a multicenter, international, non-randomized clinical trial study protocol
Read this article at
Abstract
Background
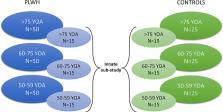
The burden of herpes zoster (shingles) virus and associated complications, such as post-herpetic neuralgia, is higher in older adults and has a significant impact on quality of life. The incidence of herpes zoster and post-herpetic neuralgia is increased in people living with HIV (PLWH) compared to an age-matched general population, including PLWH on long-term antiretroviral therapy (ART) with no detectable viremia and normal CD4 counts. PLWH – even on effective ART may- exhibit sustained immune dysfunction, as well as defects in cells involved in the response to vaccines. In the context of herpes zoster, it is therefore important to assess the immune response to varicella zoster virus vaccination in older PLWH and to determine whether it significantly differs to that of HIV-uninfected healthy adults or younger PLWH. We aim at bridging these knowledge gaps by conducting a multicentric, international, non-randomised clinical study (SHINGR’HIV) with prospective data collection after vaccination with an adjuvant recombinant zoster vaccine (RZV) in two distinct populations: in PLWH on long-term ART (> 10 years) over 50 years of and age/gender matched controls.
Methods
We will recruit participants from two large established HIV cohorts in Switzerland and in France in addition to age-/gender-matched HIV-uninfected controls. Participants will receive two doses of RZV two months apart. In depth-evaluation of the humoral, cellular, and innate immune responses and safety profile of the RZV will be performed to address the combined effect of aging and potential immune deficiencies due to chronic HIV infection. The primary study outcome will compare the geometric mean titer (GMT) of gE-specific total IgG measured 1 month after the second dose of RZV between different age groups of PLWH and between PLWH and age-/gender-matched HIV-uninfected controls.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults.
- Record: found
- Abstract: found
- Article: not found
Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older.
- Record: found
- Abstract: found
- Article: not found
Correlates of adjuvanticity: A review on adjuvants in licensed vaccines
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.