- Record: found
- Abstract: found
- Article: found
Icariside II overcomes TRAIL resistance of melanoma cells through ROS-mediated downregulation of STAT3/cFLIP signaling
Read this article at
Abstract
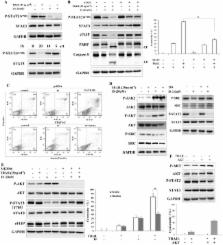
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising antitumor agent. However, many melanoma cells show weak responses to TRAIL. Here, we investigated whether Icariside II (IS), an active component of Herba Epimedii, could potentiate antitumor effects of TRAIL in melanoma cells. Melanoma cells were treated with IS and/or TRAIL and cell death, apoptosis and signal transduction were analyzed. We showed that IS promoted TRAIL-induced cell death and apoptosis in A375 melanoma cells. Mechanistically, IS reduced the expression levels of cFLIP in a phospho-STAT3 (pSTAT3)-dependent manner. Ectopic expression of STAT3 abolished IS-induced cFLIP down-regulation and the associated potentiation of TRAIL-mediated cell death. Moreover, IS-induced reactive oxygen species (ROS) production preceded down-regulation of pSTAT3/cFLIP via activating AKT, and the consequent sensitization of cells to TRAIL. We also found that IS treatment down-regulated cFLIP via ROS-mediated NF-κB pathway. In addition, IS converted TRAIL-resistant melanoma MeWo and SK-MEL-28 cells into TRAIL-sensitive cells. Taken together, our results indicated that IS potentiated TRAIL-induced apoptosis through ROS-mediated down-regulation of STAT3/cFLIP signaling.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Executioner caspase-3 and caspase-7 are functionally distinct proteases.
- Record: found
- Abstract: found
- Article: not found
Targeting JAK kinase in solid tumors: emerging opportunities and challenges.
- Record: found
- Abstract: not found
- Article: not found