- Record: found
- Abstract: found
- Article: found
Management of Chronic Graft-vs.-Host Disease in Children and Adolescents With ALL: Present Status and Model for a Personalised Management Plan
Read this article at
Abstract
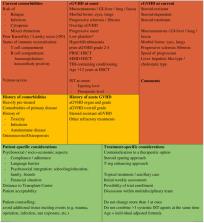
Herein we review current practice regarding the management of chronic graft-vs.-host disease (cGvHD) in paediatric patients after allogeneic haematopoietic stem cell transplantation (HSCT) for acute lymphoblastic leukaemia (ALL). Topics covered include: (i) the epidemiology of cGvHD; (ii) an overview of advances in our understanding cGvHD pathogenesis; (iii) current knowledge regarding risk factors for cGvHD and prevention strategies complemented by biomarkers; (iii) the paediatric aspects of the 2014 National Institutes for Health-defined diagnosis and grading of cGvHD; and (iv) current options for cGvHD treatment. We cover topical therapy and newly approved tyrosine kinase inhibitors, emphasising the use of immunomodulatory approaches in the context of the delicate counterbalance between immunosuppression and immune reconstitution as well as risks of relapse and infectious complications. We examine real-world approaches of response assessment and tapering schedules of treatment. Furthermore, we report on the optimal timepoints for therapeutic interventions and changes in relation to immune reconstitution and risk of relapse/infection. Additionally, we review the different options for anti-infectious prophylaxis. Finally, we put forth a theory of a holistic view of paediatric cGvHD and its associated manifestations and propose a checklist for individualised risk evaluation with aggregated considerations including site-specific cGvHD evaluation with attention to each individual's GvHD history, previous medical history, comorbidities, and personal tolerance and psychosocial circumstances. To complement this checklist, we present a treatment algorithm using representative patients to inform the personalised management plans for patients with cGvHD after HSCT for ALL who are at high risk of relapse.
Related collections
Most cited references282
- Record: found
- Abstract: found
- Article: not found
Graft-versus-host disease.
- Record: found
- Abstract: found
- Article: not found
National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report.
- Record: found
- Abstract: found
- Article: not found