- Record: found
- Abstract: found
- Article: found
Delivery of nanovaccine towards lymphoid organs: recent strategies in enhancing cancer immunotherapy
Read this article at
Graphical Abstract
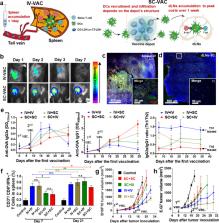
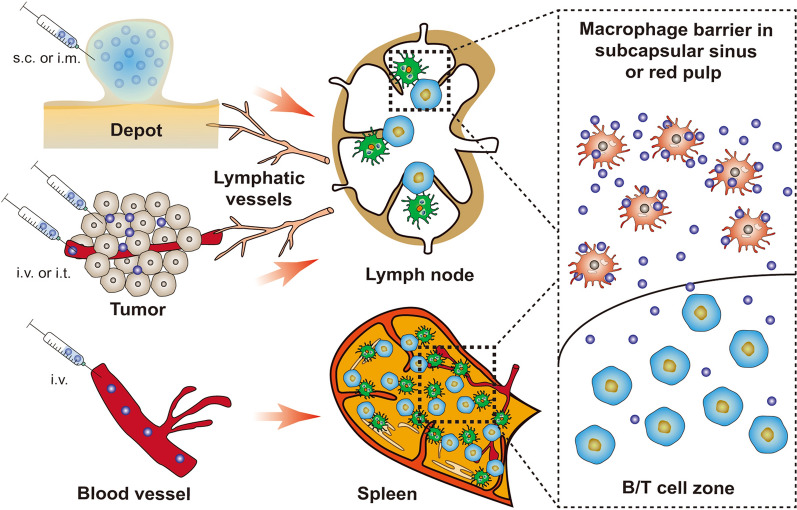
With the in-depth exploration on cancer therapeutic nanovaccines, increasing evidence shows that the poor delivery of nanovaccines to lymphoid organs has become the culprit limiting the rapid induction of anti-tumor immune response. Unlike the conventional prophylactic vaccines that mainly form a depot at the injection site to gradually trigger durable immune response, the rapid proliferation of tumors requires an efficient delivery of nanovaccines to lymphoid organs for rapid induction of anti-tumor immunity. Optimization of the physicochemical properties of nanovaccine ( e.g., size, shape, charge, colloidal stability and surface ligands) is an effective strategy to enhance their accumulation in lymphoid organs, and nanovaccines with dynamic structures are also designed for precise targeted delivery of lymphoid organs or their subregions. The recent progress of these nanovaccine delivery strategies is highlighted in this review, and the challenges and future direction are also discussed.
Related collections
Most cited references107
- Record: found
- Abstract: found
- Article: not found
Neoantigens in cancer immunotherapy.
- Record: found
- Abstract: found
- Article: not found
Tumor-associated macrophages: from mechanisms to therapy.
- Record: found
- Abstract: found
- Article: not found