- Record: found
- Abstract: not found
- Article: not found
Killers 2.0: NK cell therapies at the forefront of cancer control
September 3 2019
September 3 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Natural killer (NK) cells are innate cytotoxic lymphocytes involved in the surveillance
and elimination of cancer. As we have learned more and more about the mechanisms NK
cells employ to recognize and eliminate tumor cells, and how, in turn, cancer evades
NK cell responses, we have gained a clear appreciation that NK cells can be harnessed
in cancer immunotherapy. Here, we review the evidence for NK cells’ critical role
in combating transformed and malignant cells, and how cancer immunotherapies potentiate
NK cell responses for therapeutic purposes. We highlight cutting-edge immunotherapeutic
strategies in preclinical and clinical development such as adoptive NK cell transfer,
chimeric antigen receptor–expressing NK cells (CAR-NKs), bispecific and trispecific
killer cell engagers (BiKEs and TriKEs), checkpoint blockade, and oncolytic virotherapy.
Further, we describe the challenges that NK cells face (e.g., postsurgical dysfunction)
that must be overcome by these therapeutic modalities to achieve cancer clearance.
Related collections
Most cited references149
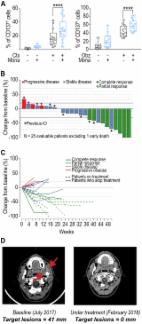
- Record: found
- Abstract: found
- Article: found
Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells
Pascale André, Caroline Denis, Caroline Soulas … (2018)
- Record: found
- Abstract: found
- Article: not found
Natural killer cells and other innate lymphoid cells in cancer
Eric Vivier, Pierre-Yves Dumas, Margaux Vienne … (2018)
- Record: found
- Abstract: found
- Article: not found
Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy.
K Kärre, H Ljunggren, G Piontek … (1986)
