- Record: found
- Abstract: found
- Article: found
Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions
Read this article at
Abstract
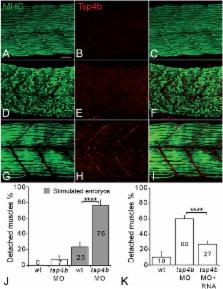
Tendons are extracellular matrix (ECM)-rich structures that mediate muscle attachments with the skeleton, but surprisingly little is known about molecular mechanisms of attachment. Individual myofibers and tenocytes in Drosophila interact through integrin (Itg) ligands such as Thrombospondin (Tsp), while vertebrate muscles attach to complex ECM fibrils embedded with tenocytes. We show for the first time that a vertebrate thrombospondin, Tsp4b, is essential for muscle attachment and ECM assembly at myotendinous junctions (MTJs). Tsp4b depletion in zebrafish causes muscle detachment upon contraction due to defects in laminin localization and reduced Itg signaling at MTJs. Mutation of its oligomerization domain renders Tsp4b unable to rescue these defects, demonstrating that pentamerization is required for ECM assembly. Furthermore, injected human TSP4 localizes to zebrafish MTJs and rescues muscle detachment and ECM assembly in Tsp4b-deficient embryos. Thus Tsp4 functions as an ECM scaffold at MTJs, with potential therapeutic uses in tendon strengthening and repair.
eLife digest
Tendons, the tough connective tissues that link muscles to bones, are essential for lifting, running and other movements in animals. A matrix of proteins, called the extracellular matrix, connects the cells in a tendon, giving it the strength it needs to prevent muscles from detaching from bones during strenuous activities.
To achieve this strength, extracellular matrix proteins bind to one another and to receptors on the muscle cell surface that are linked to its internal scaffolding, thereby organizing other proteins into a structure called a myotendinous junction. However, despite the essential roles of tendons, scientists do not fully understand how this organization occurs, or how it can go awry.
Subramanian and Schilling screened zebrafish for genes that are essential for proper muscle attachment, and zeroed in on a gene encoding a protein called Thrombospondin-4b (Tsp4b). A similar protein helps to connect muscle and tendon cells in fruit flies. Without Tsp4b, zebrafish are able to form connections between muscles and tendons, but the muscles detach easily during movement. This weakened connection is caused by disorganization of the proteins in the extracellular matrix, which results in reduced signaling from the muscle cell receptors.
When a human form of this protein was injected into zebrafish embryos lacking Tsp4b, it settled into the junctions between muscle and tendon cells. The human protein repaired the detached muscles and restored the proper organization of the matrix. This improved the strength of the muscle-tendon attachment in the treated fish embryos, suggesting that similar injections could also help to strengthen and repair muscles and tendons in people.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
RGD and other recognition sequences for integrins.
- Record: found
- Abstract: found
- Article: not found
Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis.
- Record: found
- Abstract: found
- Article: not found