- Record: found
- Abstract: found
- Article: found
Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: implications for multiple sclerosis
Read this article at
Abstract
Introduction
The inverse correlation between prevalence of auto-immune disorders like the chronic neuro-inflammatory disease multiple sclerosis (MS) and the occurrence of helminth (worm) infections, suggests that the helminth-trained immune system is protective against auto-immunity. As monocytes are regarded as crucial players in the pathogenesis of auto-immune diseases, we explored the hypothesis that these innate effector cells are prime targets for helminths to exert their immunomodulatory effects.
Results
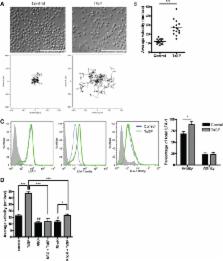
Here we show that soluble products of the porcine nematode Trichuris suis (TsSP) are potent in changing the phenotype and function of human monocytes by skewing classical monocytes into anti-inflammatory patrolling cells, which exhibit reduced trans-endothelial migration capacity in an in vitro model of the blood–brain barrier. Mechanistically, we identified the mannose receptor as the TsSP-interacting monocyte receptor and we revealed that specific downstream signalling occurs via protein kinase C (PKC), and in particular PKCδ.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Multiple sclerosis--the plaque and its pathogenesis.
- Record: found
- Abstract: found
- Article: not found