- Record: found
- Abstract: found
- Article: found
Neuroprotective effects of gemfibrozil in neurological disorders: Focus on inflammation and molecular mechanisms
Read this article at
Abstract
Background
Gemfibrozil (Gem) is a drug that has been shown to activate PPAR‐α, a nuclear receptor that plays a key role in regulating lipid metabolism. Gem is used to lower the levels of triglycerides and reduce the risk of coronary heart disease in patients. Experimental studies in vitro and in vivo have shown that Gem can prevent or slow the progression of neurological disorders (NDs), including cerebral ischemia (CI), Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis (MS). Neuroinflammation is known to play a significant role in these disorders.
Method
The literature review for this study was conducted by searching Scopus, Science Direct, PubMed, and Google Scholar databases.
Result
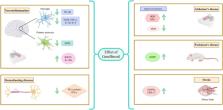
The results of this study show that Gem has neuroprotective effects through several cellular and molecular mechanisms such as: (1) Gem has the ability to upregulate pro‐survival factors (PGC‐1α and TFAM), promoting the survival and function of mitochondria in the brain, (2) Gem strongly inhibits the activation of NF‐κB, AP‐1, and C/EBPβ in cytokine‐stimulated astroglial cells, which are known to increase the expression of iNOS and the production of NO in response to proinflammatory cytokines, (3) Gem protects dopamine neurons in the MPTP mouse model of PD by increasing the expression of PPARα, which in turn stimulates the production of GDNF in astrocytes, (4) Gem reduces amyloid plaque pathology, reduces the activity of glial cells, and improves memory, (5) Gem increases myelin genes expression (MBP and CNPase) via PPAR‐β, and (6) Gem increases hippocampal BDNF to counteract depression.
Abstract
Neuroprotective effects of Gem on NDs. Gem exhibits neuroprotective properties by reducing inflammation, oxidative stress, and apoptosis in the brain. The mechanism of action involves modulating lipid metabolism, upregulating antioxidant enzymes, and inhibiting proinflammatory cytokines. Preclinical studies have shown that Gem treatment protects neurons, promotes neurite outgrowth, and improves cognitive function in animal models of neurodegenerative diseases like Alzheimer's and Parkinson's.
Related collections
Most cited references200
- Record: found
- Abstract: found
- Article: not found
From stress to inflammation and major depressive disorder: a social signal transduction theory of depression.
- Record: found
- Abstract: found
- Article: not found
A new mechanism of nervous system plasticity: activity-dependent myelination.
- Record: found
- Abstract: found
- Article: not found
