- Record: found
- Abstract: found
- Article: found
Laminin γ3 plays an important role in retinal lamination, photoreceptor organisation and ganglion cell differentiation
Read this article at
Abstract
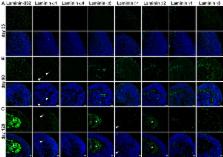
Laminins are heterotrimeric glycoproteins of the extracellular matrix. Eleven different laminin chains have been identified in vertebrates. They are ubiquitously expressed in the human body, with a distinct tissue distribution. Laminin expression in neural retina and their functional role during human retinogenesis is still unknown. This study investigated the laminin expression in human developing and adult retina, showing laminin α1, α5, β1, β2 and γ1 to be predominantly expressed in Bruch’s membrane and the inner limiting membrane. Laminin-332 and laminin γ3 expression were mainly observed in the neural retina during retinal histogenesis. These expression patterns were largely conserved in pluripotent stem cell-derived retinal organoids. Blocking of laminin γ3 function in retinal organoids resulted in the disruption of laminar organisation and synapse formation, the loss of photoreceptor organisation and retinal ganglion cells. Our data demonstrate a unique temporal and spatial expression for laminins and reveal a novel role for laminin γ3 during human retinogenesis.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Functional diversity of laminins.
- Record: found
- Abstract: found
- Article: not found