- Record: found
- Abstract: found
- Article: found
Anticancer effects of 4-vinyl-2,6-dimethoxyphenol (canolol) against SGC-7901 human gastric carcinoma cells
Read this article at
Abstract
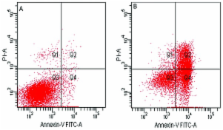
Gastric cancer remains the fourth most commonly diagnosed cancer and is the second leading cause of cancer-related mortality worldwide. The aim of this study was to investigate the effects of canolol on the proliferation and apoptosis of SGC-7901 human gastric cancer cells and its relevant molecular mechanisms. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to observe the effect of canolol on the proliferation of SGC-7901 human gastric adenocarcinoma cells. The results showed that SGC-7901 cells exhibited a marked dose-dependent reduction in the proliferation rate. The survival rate of the cells was 88.86±1.58% at 50 μmol/l, decreasing to 53.73±1.51% at 800 μmol/l (P<0.05). By contrast, canolol had no significant toxicity on the human gastric mucosal epithelial cell line GES-1. The vivid images of cell morphology using an inverted microscope provided confirmation of the MTT assay. Treatment of SGC-7901 cells with canolol resulted in apoptosis demonstrated by flow cytometry. Furthermore, canolol downregulated the mRNA levels of COX-2, but had no significant effect on the mRNA expession of the Bax and Bcl-2 genes. These findings suggest that canolol has potential to be developed as a new natural anti-gastric carcinoma agent.
Related collections
Most cited references33
- Record: found
- Abstract: not found
- Article: not found
Cell death in development.
- Record: found
- Abstract: found
- Article: not found
Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice.
- Record: found
- Abstract: found
- Article: not found