- Record: found
- Abstract: found
- Article: found
Downregulation of miR-205 contributes to epithelial–mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1–RAGE signaling pathway
Read this article at
Abstract
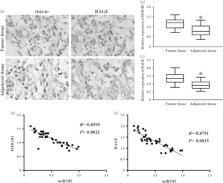
Our aim was to study the regulatory molecule networks involved in the epithelial-to-mesenchymal transition and thus promoting the early onset of metastasis in triple-negative breast cancer (TNBC). Forty pairs of human TNBC and their adjacent normal breast tissues were analyzed by real-time PCR and immunochemistry to demonstrate the correlation between the miR-205 expression and clinicopathological characteristics. In vitro, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2- H-tetrazolium bromide assay, cell migration, and invasion assay were used to detect the cell growth and invasive ability of TNBC cells after upregulation or downregulation of miR-205 expression. Luciferase reporter assay was used to confirm the potential target directly influenced by miR-205. Our results showed that miR-205 abnormal expression may be involved and associated with the biological traits of TNBC. Ectopic expression of miR-205 not only inhibited cell growth, but also suppressed migration and invasion of mesenchymal-like TNBC cells. In addition, we found that overexpression of miR-205 significantly suppressed HMGB1 by binding its 3′-untranslated region, and that miR-205 was inversely correlated with the expression of HMGB1 and RAGE in cell lines and clinical samples. Our study illustrated that miR-205 was a tumor suppressor in TNBC, which attenuated the viability and the acquisition of the epithelial-to-mesenchymal transition phenotype TNBC cells at least partially exerted through targeting of HMGB1–RAGE signaling pathway.
Related collections
Most cited references26

- Record: found
- Abstract: found
- Article: found
Triple-negative breast cancer
- Record: found
- Abstract: found
- Article: not found
A review of triple-negative breast cancer.
- Record: found
- Abstract: found
- Article: found