- Record: found
- Abstract: found
- Article: not found
Corowa-kun: A messenger app chatbot delivers COVID-19 vaccine information, Japan 2021
Abstract
Background
There is a long history in Japan of public concerns about vaccine adverse events. Few studies have assessed how mobile messenger apps affect COVID-19 vaccine hesitancy.
Methods
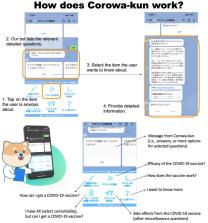
Corowa-kun, a free chatbot, was created on February 6, 2021 in LINE, the most popular messenger app in Japan. Corowa-kun provides instant, automated answers to 70 frequently asked COVID-19 vaccine questions. A cross-sectional survey with 21 questions was performed within Corowa-kun during April 5–12, 2021.
Results
A total of 59,676 persons used Corowa-kun during February–April 2021. Of them, 10,192 users (17%) participated in the survey. Median age was 55 years (range 16–97), and most were female (74%). COVID-19 vaccine hesitancy reported by survey respondents decreased from 41% to 20% after using Corowa-kun. Of the 20% who remained hesitant, 16% (1,675) were unsure, and 4% (364) did not intend to be vaccinated. Factors associated with vaccine hesitancy were: age 16−34 (odds ratio [OR] = 3.7; 95% confidential interval [CI]: 3.0–4.6, compared to age ≥65), female sex (OR = 2.4; Cl: 2.1–2.8), and history of a previous vaccine side-effect (OR = 2.5; Cl: 2.2–2.9). Being a physician (OR = 0.2; Cl: 0.1−0.4) and having received a flu vaccine the prior season (OR = 0.4; Cl: 0.3–0.4) were protective.
Conclusions
A substantial number of people used the chabot in a short period. Mobile messenger apps could be leveraged to provide accurate vaccine information and to investigate vaccine intention and risk factors for vaccine hesitancy.
Text (2874 of 3500)
Background
In 2020, a global coronavirus disease-2019 (COVID-19) pandemic emerged, caused by the SARS-CoV-2 virus. [1] According to the World Health Organization, there have been 516 million confirmed cases of COVID-19, including 6 million deaths (as of May 11, 2022). [2] Multiple COVID-19 vaccines are highly effective at preventing symptomatic disease. [3] Most developed countries have already vaccinated large proportions of their populations. However, many individuals choose not to be vaccinated, often citing safety concerns. Early in the pandemic, studies revealed high levels of COVID-19 vaccine hesitancy, ranging from 20−40% of the surveyed populations. [4], [5], [6], [7], [8] Vaccine hesitancy differs depending on sociodemographic factors, such as race and educational level, as well as attitudes and beliefs. [9], [10], [11], [12], [13] Understanding peoples’ concerns about COVID-19 vaccines is necessary to increase vaccine uptake among those hesitant.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
A Novel Coronavirus from Patients with Pneumonia in China, 2019
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: not found
A global survey of potential acceptance of a COVID-19 vaccine
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.