- Record: found
- Abstract: found
- Article: found
CONNECT for quality: protocol of a cluster randomized controlled trial to improve fall prevention in nursing homes
Read this article at
Abstract
Background
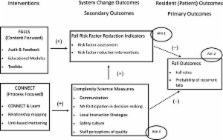
Quality improvement (QI) programs focused on mastery of content by individual staff members are the current standard to improve resident outcomes in nursing homes. However, complexity science suggests that learning is a social process that occurs within the context of relationships and interactions among individuals. Thus, QI programs will not result in optimal changes in staff behavior unless the context for social learning is present. Accordingly, we developed CONNECT, an intervention to foster systematic use of management practices, which we propose will enhance effectiveness of a nursing home Falls QI program by strengthening the staff-to-staff interactions necessary for clinical problem-solving about complex problems such as falls. The study aims are to compare the impact of the CONNECT intervention, plus a falls reduction QI intervention (CONNECT + FALLS), to the falls reduction QI intervention alone (FALLS), on fall-related process measures, fall rates, and staff interaction measures.
Methods/design
Sixteen nursing homes will be randomized to one of two study arms, CONNECT + FALLS or FALLS alone. Subjects (staff and residents) are clustered within nursing homes because the intervention addresses social processes and thus must be delivered within the social context, rather than to individuals. Nursing homes randomized to CONNECT + FALLS will receive three months of CONNECT first, followed by three months of FALLS. Nursing homes randomized to FALLS alone receive three months of FALLs QI and are offered CONNECT after data collection is completed. Complexity science measures, which reflect staff perceptions of communication, safety climate, and care quality, will be collected from staff at baseline, three months after, and six months after baseline to evaluate immediate and sustained impacts. FALLS measures including quality indicators (process measures) and fall rates will be collected for the six months prior to baseline and the six months after the end of the intervention. Analysis will use a three-level mixed model.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Missing data: our view of the state of the art.
- Record: found
- Abstract: found
- Article: not found
Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.
- Record: found
- Abstract: found
- Article: not found