- Record: found
- Abstract: found
- Article: found
The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows
Read this article at
Abstract
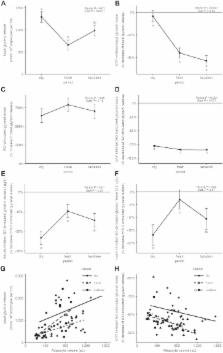
Hormone sensitive lipase (HSL) activation is part of the metabolic adaptations to the negative energy balance common to the mammalian periparturient period. This study determined HSL contribution to adipose tissue (AT) lipolysis and how insulin regulates its activity in periparturient dairy cows. Subcutaneous AT (SCAT) samples were collected at 11 d prepartum (dry) and 11 (fresh) and 24 d (lactation) postpartum. Basal and stimulated lipolysis (ISO) responses were determined using explant cultures. HSL contribution to lipolysis was assessed using an HSL inhibitor (CAY). Basal lipolysis was higher in SCAT at dry compared with fresh. CAY inhibited basal lipolysis negligibly at dry, but at fresh and lactation it reduced basal lipolysis by 36.1 ± 4.51% and 43.1 ± 4.83%, respectively. Insulin inhibited lipolysis more pronouncedly in dry compared to fresh. Results demonstrate that HSL contribution to basal lipolysis is negligible prepartum. However, HSL is a major driver of SCAT lipolytic responses postpartum. Lower basal lipolysis postpartum suggests that reduced lipogenesis is an important contributor to fatty acid release from SCAT. Loss of adipocyte sensitivity to the antilipolytic action of insulin develops in the early lactation period and supports a state of insulin resistance in AT of cows during the first month postpartum.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus.
- Record: found
- Abstract: found
- Article: not found
Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores.
- Record: found
- Abstract: found
- Article: not found