- Record: found
- Abstract: found
- Article: found
Still in Search for an EAAT Activator: GT949 Does Not Activate EAAT2, nor EAAT3 in Impedance and Radioligand Uptake Assays
Read this article at
Abstract

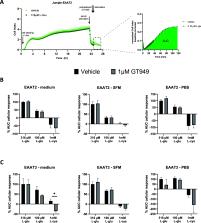
Excitatory amino acid transporters (EAATs) are important regulators of amino acid transport and in particular glutamate. Recently, more interest has arisen in these transporters in the context of neurodegenerative diseases. This calls for ways to modulate these targets to drive glutamate transport, EAAT2 and EAAT3 in particular. Several inhibitors (competitive and noncompetitive) exist to block glutamate transport; however, activators remain scarce. Recently, GT949 was proposed as a selective activator of EAAT2, as tested in a radioligand uptake assay. In the presented research, we aimed to validate the use of GT949 to activate EAAT2-driven glutamate transport by applying an innovative, impedance-based, whole-cell assay (xCELLigence). A broad range of GT949 concentrations in a variety of cellular environments were tested in this assay. As expected, no activation of EAAT3 could be detected. Yet, surprisingly, no biological activation of GT949 on EAAT2 could be observed in this assay either. To validate whether the impedance-based assay was not suited to pick up increased glutamate uptake or if the compound might not induce activation in this setup, we performed radioligand uptake assays. Two setups were utilized; a novel method compared to previously published research, and in a reproducible fashion copying the methods used in the existing literature. Nonetheless, activation of neither EAAT2 nor EAAT3 could be observed in these assays. Furthermore, no evidence of GT949 binding or stabilization of purified EAAT2 could be observed in a thermal shift assay. To conclude, based on experimental evidence in the present study GT949 requires specific assay conditions, which are difficult to reproduce, and the compound cannot simply be classified as an activator of EAAT2 based on the presented evidence. Hence, further research is required to develop the tools needed to identify new EAAT modulators and use their potential as a therapeutic target.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Characterization of novel L-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters.
- Record: found
- Abstract: found
- Article: not found
The importance of the excitatory amino acid transporter 3 (EAAT3).
- Record: found
- Abstract: not found
- Article: not found