- Record: found
- Abstract: found
- Article: found
Toxoplasma gondii Rhoptry Protein ROP16 Mediates Partially SH-SY5Y Cells Apoptosis and Cell Cycle Arrest by Directing Ser15/37 Phosphorylation of p53
Read this article at
Abstract
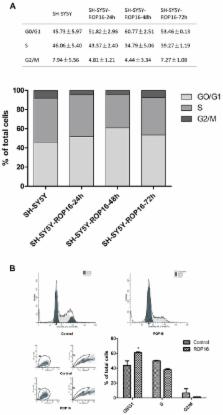
Toxoplasma rhoptries, an unusual set of apical organelles that are associated with Toxoplasma infection may cause subversion of the host cell functions. Parasite rhoptry protein 16 (ROP16) is a regulator of host cell transcription during cell invasion in which it migrates into the host cell cytoplasm and subsequently localizes to the nucleus. In the present study, we found that overexpression of ROP16 could partially mediate human neuroblastoma SH-SY5Y apoptosis (12.47%) and cell cycle arrest in G1 phase (60.77%) in a p53 dependent manner by influencing the expression of Bax/Bcl-2 and p21/CDKs. ROP16 was identified to co-localize with p53, a novel direct interaction partner in the nucleus of SH-SY5Y. Furthermore, SH-SY5Y apoptosis via the mitochondria-dependent p53 pathway and cell cycle arrest caused by ROP16 dealt with direct serine 15/37 phosphorylation of p53. Our studies provide a new mechanism by which ROP16 interacts with the nucleus proteins which subsequently subverts the host cells functions.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53.
- Record: found
- Abstract: found
- Article: not found
