- Record: found
- Abstract: found
- Article: found
BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis
Read this article at
Abstract
Background
Patients with interstitial cystitis/bladder pain syndrome (IC/BPS) often grieve over a low quality of life brought about by chronic pain. In our previous studies, we determined that neuroinflammation of the spinal dorsal horn (SDH) was associated with mechanisms of interstitial cystitis. Moreover, it has been shown that brain-derived neurotrophic factor (BDNF) participates in the regulation of neuroinflammation and pathological pain through BDNF-TrkB signaling; however, whether it plays a role in cyclophosphamide (CYP)-induced cystitis remains unclear. This study aimed to confirm whether BDNF-TrkB signaling modulates neuroinflammation and mechanical allodynia in CYP-induced cystitis and determine how it occurs.
Methods
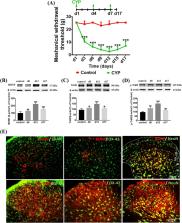
Systemic intraperitoneal injection of CYP was performed to establish a rat cystitis model. BDNF-TrkB signaling was modulated by intraperitoneal injection of the TrkB receptor antagonist, ANA-12, or intrathecal injection of exogenous BDNF. Mechanical allodynia in the suprapubic region was assessed using the von Frey filaments test. The expression of BDNF, TrkB, p-TrkB, Iba1, GFAP, p-p38, p-JNK, IL-1β, and TNF-α in the L6-S1 SDH was measured by Western blotting and immunofluorescence analysis.
Results
BDNF-TrkB signaling was upregulated significantly in the SDH after CYP was injected. Similarly, the expressions of Iba1, GFAP, p-p38, p-JNK, IL-1β, and TNF-α in the SDH were all upregulated. Treatment with ANA-12 could attenuate mechanical allodynia, restrain activation of astrocytes and microglia and alleviate neuroinflammation. Besides, the intrathecal injection of exogenous BDNF further decreased the mechanical withdrawal threshold, promoted activation of astrocytes and microglia, and increased the release of TNF-α and IL-1β in the SDH of our CYP-induced cystitis model.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Neuroinflammation in the central nervous system: Symphony of glial cells
- Record: found
- Abstract: found
- Article: not found
Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice.

- Record: found
- Abstract: found
- Article: found