- Record: found
- Abstract: found
- Article: found
Circulating Lactonase Activity but Not Protein Level of PON-1 Predicts Adverse Outcomes in Subjects with Chronic Kidney Disease
Read this article at
Abstract
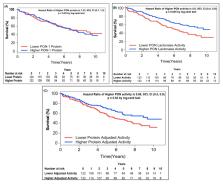
The burden of cardiovascular disease and death in chronic kidney disease (CKD) outpaces that of the other diseases and is not adequately described by traditional risk factors alone. Diminished activity of paraoxonase (PON)-1 is associated with increased oxidant stress, a common feature underlying the pathogenesis of CKD. We aimed to assess the prognostic value of circulating PON-1 protein and PON lactonase activity on adverse clinical outcomes across various stages and etiologies of CKD. Circulating PON-1 protein levels and PON lactonase activity were measured simultaneously in patients with CKD as well as a cohort of apparently healthy non-CKD subjects. Both circulating PON-1 protein levels and PON lactonase activity were significantly lower in CKD patients compared to the non-CKD subjects. Similarly, across all stages of CKD, circulating PON-1 protein and PON lactonase activity were significantly lower in patients with CKD compared to the non-CKD controls. Circulating PON lactonase activity, but not protein levels, predicted future adverse clinical outcomes, even after adjustment for traditional risk factors. The combination of lower circulating protein levels and higher activity within the CKD subjects were associated with the best survival outcomes. These findings demonstrate that diminished circulating PON lactonase activity, but not protein levels, predicts higher risk of future adverse clinical outcomes in patients with CKD.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities.

- Record: found
- Abstract: found
- Article: found
Antioxidant and Anti-Inflammatory Role of Paraoxonase 1: Implication in Arteriosclerosis Diseases
- Record: found
- Abstract: found
- Article: found