- Record: found
- Abstract: found
- Article: found
The marine-derived HIF-1α inhibitor, Yardenone 2, reduces prostate cancer cell proliferation by targeting HIF-1 target genes
Read this article at
Abstract
Background
Prostate cancer (PCa) ranks as the second most prevalent cancer in men, with advanced stages posing significant treatment challenges. Given its solid tumor nature, PCa is highly susceptible to hypoxia, a condition associated with resistance to radiation and chemotherapy, metastasis, and unfavorable patient outcomes. Hypoxia-inducible factors (HIFs) play a pivotal role in cancer cell adaptation to hypoxic environments, contributing to treatment resistance. Consequently, inhibitors targeting HIFs hold promise for cancer therapy.
Methods
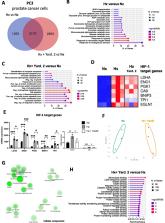
In this study, we aimed to characterize novel HIF-1α inhibitors including Sodwanones A (1), B (2), C (3), G (4) and Yardenone 2 (5) isolated from marine sponges belonging to the Axinella genus. Our investigation evaluated the impact of these compounds on various aspects of HIF-1α regulation, including stabilization, nuclear localization, expression of HIF-1 target genes (while sparing HIF-2 target genes), cellular metabolism, as well as cell proliferation and viability in prostate cells under hypoxic conditions.
Results
Our findings revealed that among the compounds tested, Yardenone 2 exhibited notable effects in hypoxia: it destabilized HIF-1α at the protein level, decreased its nuclear localization, selectively altered the expression of HIF-1 target genes, and restrained cell proliferation in aggressive PC3 prostate cancer cells as well as in an MSK-PCa3 patient-derived organoid line. Moreover, it affected the morphology of these organoid. Yardenone 2 was also compared to Docetaxel, a specific microtubule inhibitor and a drug used in the treatment of prostate cancer. The comparison between the two compounds revealed notable differences, such as a lack of specificity to hypoxic cells of Docetaxel.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Targeting HIF-1 for cancer therapy.
- Record: found
- Abstract: found
- Article: not found
Organoid cultures derived from patients with advanced prostate cancer.
- Record: found
- Abstract: found
- Article: not found