- Record: found
- Abstract: found
- Article: found
Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease
Read this article at
Summary
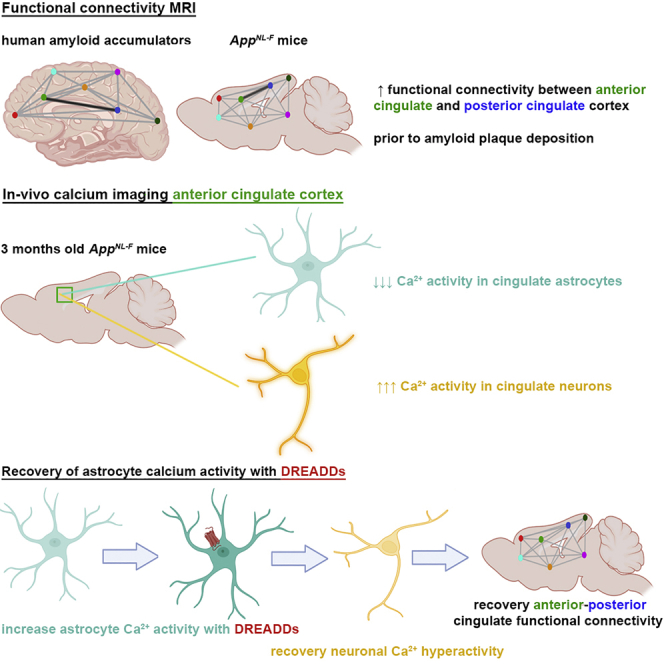
Dysfunctions of network activity and functional connectivity (FC) represent early events in Alzheimer’s disease (AD), but the underlying mechanisms remain unclear. Astrocytes regulate local neuronal activity in the healthy brain, but their involvement in early network hyperactivity in AD is unknown. We show increased FC in the human cingulate cortex several years before amyloid deposition. We find the same early cingulate FC disruption and neuronal hyperactivity in App NL-F mice. Crucially, these network disruptions are accompanied by decreased astrocyte calcium signaling. Recovery of astrocytic calcium activity normalizes neuronal hyperactivity and FC, as well as seizure susceptibility and day/night behavioral disruptions. In conclusion, we show that astrocytes mediate initial features of AD and drive clinically relevant phenotypes.
Graphical abstract
Highlights
-
•
The cingulate cortex of humans and mice shows early functional deficits in AD
-
•
Astrocyte calcium signaling is decreased before the presence of amyloid plaques
-
•
Recovery of astrocyte calcium signals mitigates neuronal hyperactivity
-
•
Recovery of astrocytes normalizes cingulate connectivity and behavior disruptions
Abstract
Shah et al. show disrupted cingulate functional connectivity in mice and humans before amyloid deposition. Cingulate astrocytes demonstrate decreased calcium signaling, while neurons are hyperactive. Restoring the regulatory activity of astrocytes recovers neuronal hyperactivity, cingulate connectivity, and behavior deficits, suggesting that astrocytes represent a major player in early Alzheimer’s disease.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain.
- Record: found
- Abstract: found
- Article: found