- Record: found
- Abstract: found
- Article: found
Transgenesis for pig models
Read this article at
Abstract
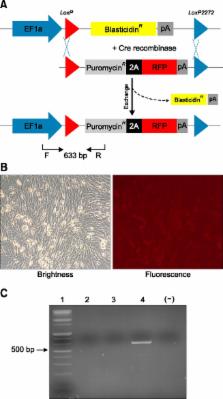
Animal models, particularly pigs, have come to play an important role in translational biomedical research. There have been many pig models with genetically modifications via somatic cell nuclear transfer (SCNT). However, because most transgenic pigs have been produced by random integration to date, the necessity for more exact gene-mutated models using recombinase based conditional gene expression like mice has been raised. Currently, advanced genome-editing technologies enable us to generate specific gene-deleted and -inserted pig models. In the future, the development of pig models with gene editing technologies could be a valuable resource for biomedical research.
Related collections
Most cited references37
- Record: found
- Abstract: not found
- Article: not found
Establishment in culture of pluripotential cells from mouse embryos.
- Record: found
- Abstract: found
- Article: not found
Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos.
- Record: found
- Abstract: found
- Article: not found