- Record: found
- Abstract: found
- Article: found
MUSCLEMOTION : A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo
Read this article at
Abstract
Supplemental Digital Content is available in the text.
Abstract
Rationale:
There are several methods to measure cardiomyocyte and muscle contraction, but these require customized hardware, expensive apparatus, and advanced informatics or can only be used in single experimental models. Consequently, data and techniques have been difficult to reproduce across models and laboratories, analysis is time consuming, and only specialist researchers can quantify data.
Objective:
Here, we describe and validate an automated, open-source software tool (MUSCLEMOTION) adaptable for use with standard laboratory and clinical imaging equipment that enables quantitative analysis of normal cardiac contraction, disease phenotypes, and pharmacological responses.
Methods and Results:
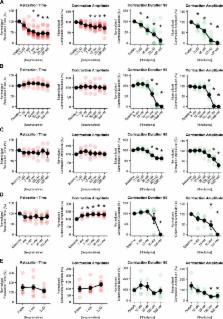
MUSCLEMOTION allowed rapid and easy measurement of movement from high-speed movies in (1) 1-dimensional in vitro models, such as isolated adult and human pluripotent stem cell-derived cardiomyocytes; (2) 2-dimensional in vitro models, such as beating cardiomyocyte monolayers or small clusters of human pluripotent stem cell-derived cardiomyocytes; (3) 3-dimensional multicellular in vitro or in vivo contractile tissues, such as cardiac “organoids,” engineered heart tissues, and zebrafish and human hearts. MUSCLEMOTION was effective under different recording conditions (bright-field microscopy with simultaneous patch-clamp recording, phase contrast microscopy, and traction force microscopy). Outcomes were virtually identical to the current gold standards for contraction measurement, such as optical flow, post deflection, edge-detection systems, or manual analyses. Finally, we used the algorithm to quantify contraction in in vitro and in vivo arrhythmia models and to measure pharmacological responses.
Related collections
Most cited references19

- Record: found
- Abstract: found
- Article: found
Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function
- Record: found
- Abstract: found
- Article: not found