- Record: found
- Abstract: found
- Article: not found
Recombinant human ACE2: potential therapeutics of SARS-CoV-2 infection and its complication
research-article
24 June 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Dear Editor,
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
has caused a pneumonia outbreak in Wuhan city, China, followed by global spread [1,
2]. As of 9 April, 2020, millions of confirmed cases of SARS-CoV-2 infection have
been reported, and the global death toll of SARS-CoV-2 infection has surged to tens
of thousands of victims, making it a public health emergency of international concern
(PHEIC). However, no specific antiviral drug or vaccine for SARS-CoV-2 treatment exists.
The high infectivity and the increasing fatality of SARS-CoV-2 highlight the demand
for drug discovery. SARS-CoV-2 is closely related to severe acute respiratory syndrome
coronavirus (SARS-CoV) [2]. Full-genome sequencing analysis indicated that SARS-CoV-2
shares a high-sequence identity with SARS-CoV [3]. The spike protein (S-protein) of
coronaviruses interacts with cell receptors to mediate viral entry into target cells
[4]. Additional evidence suggests that both SARS-CoV and SARS-CoV-2 employ angiotensin-converting
enzyme 2 (ACE2) as the entry receptor and that the receptor-binding domain (RBD) of
the S-protein directly binds to ACE2, triggering endocytosis of virus particles [5–7].
A recent study suggested that the binding affinity between ACE2 and the RBD of SARS-CoV-2
is 10–20 times stronger than that with the RBD of SARS-CoV [5], which likely explains
the increased infectivity of SARS-CoV-2.
ACE2 is not only a functional receptor of coronaviruses, but also acts as an important
negative regulator of the renin–angiotensin system (RAS) through conversion of the
vasoconstrictor angiotensin II (Ang II) to its metabolite angiotensin-(1–7) (Ang 1–7)
and angiotensin I(Ang I) to angiotensin-(1–9) (Ang 1–9) [7–9]. The ACE2/Ang 1–7 axis
plays a series of roles in the improvement of endothelial dysfunction, anti-inflammation,
anti-hypertension, anti-thrombus, and anti-fibrosis activity, and cardiovascular protection
[10–14]. The protective effect of ACE2 is associated with attenuating Ang II levels
and increasing Ang 1–7 levels in lung pathophysiology [10]. Emerging evidence has
shown that RAS signaling and ACE2 play crucial roles in SARS-CoV-induced acute respiratory
distress syndrome (ARDS) and lethal avian influenza A(H5N1, H7N9)-induced acute lung
injury (ALI) [14, 15]. According to pathological findings, SARS-CoV-2 is also associated
with lung failure and ARDS [16], and the majority of severely ill patients with SARS-CoV-2
infection have underlying comorbidities, such as cardiovascular disease, diabetes,
and cerebrovascular disease [1]. The anti-trypanosomal agent diminazene aceturate
(DIZE) was reported to be an ACE2 activator, which has a structure similar to that
of the established ACE2 activator xanthenone [17, 18]. DIZE was suggested to exert
protective effects in cardiovascular disease through modulating ACE2 activation and
expression to increase Ang 1–7 production and improve vascular function [17]. Owing
to the role of ACE2 in the entry of SARS-CoV-2, the upregulated expression of ACE2
had an unwanted effect. Therefore, DIZE is not suggested to be applied in the treatment
of SARS-CoV-2 infection. However, the addition of exogenous ACE2 could be a potential
treatment for SARS-CoV-2 infection, which might not only restrain the spread of SARS-CoV-2
by blocking its interaction with ACE2 on the host cell, but also modulate RAS to treat
SARS-CoV-2-related underlying comorbidities and protect the lung from developing ARDS.
Given that ACE2 is generated mainly in Clara cells and type II alveolar epithelial
cells, the production of ACE2 is severely impaired after epithelial injury in the
development of ARDS [19]. In addition, the expression of ACE2 is also severely decreased
in patients with pulmonary fibrosis [20]. Therefore, injection of recombinant human
ACE2 (rhACE2) is currently considered for treating ARDS and pulmonary arterial hypertension
[21]. Circulatory levels of ACE2 activity were markedly increased by rhACE2, which
further effectively lowered Ang II levels and generated Ang 1–7 from Ang II (Fig. 1).
Although Ang II receptor and ACE blockage were also effective in lung failure in animal
models, this treatment could cause potential adverse effects, causing systemic hypotension
in humans [22]. As shown in Fig. 1, rhACE2 also acts as a potential therapy for hypertension,
heart failure, kidney injury, and liver fibrosis [22–24].
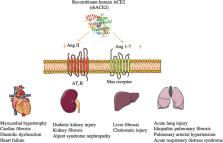
Fig. 1
The mechanism and functions of rhACE2.
rhACE2 is able to lower Ang II levels and increase Ang 1–7 levels effectively and
exert protective effects in the heart, lung, liver, and kidney.
Currently, phase I (NCT00886353) and phase II (NCT01597635) clinical studies with
a recombinant version of the catalytic ectodomain of human ACE2 (GSK2586881) have
been successfully completed, providing safety and efficacy for ARDS treatment [25,
26]. The administration of rhACE2 was well tolerated without clinically significant
hemodynamic changes in healthy subjects and patients with ARDS [26]. During the administration
period, no antibodies to rhACE2 were detected, and no serious adverse events were
reported [25]. The twice-daily doses of GSK2586881 treatment-regulated angiotensin
system peptide, leading to a significant reduction in the concentration of Ang II,
accompanied by a rapid rise in Ang 1–7 and Ang 1–5 concentrations, and caused a reduction
in IL-6 concentration [26]. However, given the small cohort of critically ill patients,
infusion of GSK2586881 did not contribute to ameliorated ARDS through physiological
or clinical measures, and a clear role of GSK2586881 in the increased reports of adverse
events referring to hypernatremia, pneumonia, dysphagia, and rash was difficult to
establish. Therefore, to assess clinical outcomes powerfully, further clinical trials
need a larger sample size. Recently, Monteil et al. [27] reported that hrACE2 could
significantly inhibit SARS-CoV-2 infection of Vero-E6 cells, and of human capillary
and kidney organoids, providing an evidence that rhACE2 might not only reduce lung
injury but also block early entry of SARS-CoV-2 infections in target cells. Further
studies are needed to illuminate the effect of hrACE2 in SARS-CoV-2 infections from
bench to clinic. To ensure the quality of the data and clinical success of rhACE2,
the trials for using rhACE2 in patients with SARS-CoV-2 infection or ARDS should consider
the patient’s stratification and continuous infusion dose. First, various plasma Ang
II levels may pose some difficulties in identifying responders. Hence, before GSK2586881
infusion, the Ang II concentrations and the ratio of ACE2/ACE activity of enrolled
patients were evaluated for improved risk stratification. ACE gene insertion/deletion
(I/D) polymorphisms play an important role in the development of hypertension, nephritis,
and cardiovascular diseases in different ethnic populations by influencing ACE and
Ang II activities [28, 29]. Identifying the specific population that is most likely
to benefit from rhACE2 represents a bright prospect. Second, due to the short half-life
of soluble ACE2 in vivo, a continuous infusion of rhACE2 may enhance efficacy. In
addition, an excess of rhACE2 is likely to influence the balance of the RAS; therefore,
it is important to identify the effective infusion dose to prevent underlying RAS-related
adverse events. Recently, it was reported that a chimeric fusion of rhACE2 and IgG2
Fc fragments could improve rhACE2 plasma stability [22]. This rhACE2-Fc fusion protein
retained full peptidase activity and had extended plasma half-life in mice [24]. The
strategy for rhACE2-Fc will be expected to provide patients with added convenience,
largely reducing administration frequency and greatly improving treatment effectiveness
[30]. Taken together, these findings indicate that rhACE2 would represent a potential
therapeutic strategy for SARS-CoV-2 infection and its complications.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
A Novel Coronavirus from Patients with Pneumonia in China, 2019
Na Zhu, Dingyu Zhang, Wenling Wang … (2020)

- Record: found
- Abstract: found
- Article: found
A pneumonia outbreak associated with a new coronavirus of probable bat origin
Peng Zhou, Xing-Lou Yang, Xian-Guang Wang … (2020)
- Record: found
- Abstract: found
- Article: not found
Pathological findings of COVID-19 associated with acute respiratory distress syndrome
Zhe Xu, Lei Shi, Yijin Wang … (2020)
