- Record: found
- Abstract: found
- Article: found
Osthole Attenuates Bleomycin-Induced Pulmonary Fibrosis by Modulating NADPH Oxidase 4-Derived Oxidative Stress in Mice
Read this article at
Abstract
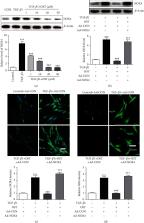
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease characterized by the extensive accumulation of myofibroblasts and collagens. However, the exact mechanism that underlies this condition is unclear. Growing evidence suggests that NADPH oxidases (NOXs), especially NOX4-derived oxidative stress, play an important role in the development of lung fibrosis. Bleomycin (BLM) is a tumor chemotherapeutic agent, which has been widely employed to establish IPF animal models. Osthole (OST) is an active constituent of the fruit of Cnidium ninidium. Here, we used an in vivo mouse model and found that OST suppressed BLM-induced body weight loss, lung injury, pulmonary index increase, fibroblast differentiation, and pulmonary fibrosis. OST also significantly downregulated BLM-induced NOX4 expression and oxidative stress in the lungs. In vitro, OST could inhibit TGF- β1-induced Smad3 phosphorylation, differentiation, proliferation, collagen synthesis, NOX4 expression, and ROS generation in human lung fibroblasts in a concentration-dependent manner. Moreover, NOX4 overexpression could prevent the above effects of OST. We came to the conclusion that OST could significantly attenuate BLM-induced pulmonary fibrosis in mice, via the mechanism that involved downregulating TGF- β1/NOX4-mediated oxidative stress in lung fibroblasts.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals.
- Record: found
- Abstract: found
- Article: not found
NADPH Oxidase-4 Mediates Myofibroblast Activation and Fibrogenic Responses to Lung Injury

- Record: found
- Abstract: found
- Article: found