- Record: found
- Abstract: found
- Article: found
Complementing the Cancer-Immunity Cycle
Read this article at
Abstract
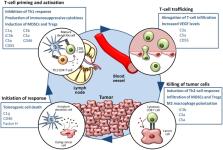
Reactivation of cytotoxic CD8 + T-cell responses has set a new direction for cancer immunotherapy. Neutralizing antibodies targeting immune checkpoint programmed cell death protein 1 (PD-1) or its ligand (PD-L1) have been particularly successful for tumor types with limited therapeutic options such as melanoma and lung cancer. However, reactivation of T cells is only one step toward tumor elimination, and a substantial fraction of patients fails to respond to these therapies. In this context, combination therapies targeting more than one of the steps of the cancer-immune cycle may provide significant benefits. To find the best combinations, it is of upmost importance to understand the interplay between cancer cells and all the components of the immune response. This review focuses on the elements of the complement system that come into play in the cancer-immunity cycle. The complement system, an essential part of innate immunity, has emerged as a major regulator of cancer immunity. Complement effectors such as C1q, anaphylatoxins C3a and C5a, and their receptors C3aR and C5aR1, have been associated with tolerogenic cell death and inhibition of antitumor T-cell responses through the recruitment and/or activation of immunosuppressive cell subpopulations such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), or M2 tumor-associated macrophages (TAMs). Evidence is provided to support the idea that complement blocks many of the effector routes associated with the cancer-immunity cycle, providing the rationale for new therapeutic combinations aimed to enhance the antitumor efficacy of anti-PD-1/PD-L1 checkpoint inhibitors.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition.

- Record: found
- Abstract: found
- Article: found
Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression
- Record: found
- Abstract: found
- Article: not found