- Record: found
- Abstract: found
- Article: found
Exercise training in pulmonary arterial hypertension associated with connective tissue diseases
Read this article at
Abstract
Introduction
The objective of this prospective study was to assess short- and long-term efficacy of exercise training (ET) as add-on to medical therapy in patients with connective tissue disease-associated pulmonary arterial hypertension (CTD-APAH).
Methods
Patients with invasively confirmed CTD-APAH received ET in-hospital for 3 weeks and continued at home for 12 weeks. Efficacy parameters have been evaluated at baseline and after 15 weeks by blinded-observers. Survival rate has been evaluated in a follow-up period of 2.9 ± 1.9 years.
Results
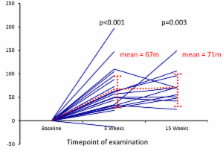
Twenty-one consecutive patients were included and assessed at baseline, and after 3 weeks, 14 after 15 weeks. Patients significantly improved the mean distance walked in 6 minutes compared to baseline by 67 ± 52 meters after 3 weeks (p < 0.001) and by 71 ± 35 meters after 15 weeks (p = 0.003), scores of quality of life (p < 0.05), heart rate at rest, peak oxygen consumption, oxygen saturation and maximal workload. Systolic pulmonary artery pressure and diastolic systemic blood pressure improved significantly after 3 weeks of ET. The 1- and 2-year overall-survival rates were 100%, the 3-year survival 73%. In one patient lung transplantation was performed 6 months after ET.
Conclusion
ET as add-on to medical therapy is highly effective in patients with CTD-APAH to improve work capacity, quality of life and further prognostic relevant parameters and possibly improves the 1-, 2- and 3-year survival rate. Further randomized controlled studies are needed to confirm these results.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2.
- Record: found
- Abstract: found
- Article: not found
Effect of encouragement on walking test performance.
- Record: found
- Abstract: found
- Article: not found