- Record: found
- Abstract: found
- Article: not found
Predictive Low-Glucose Insulin Suspension Reduces Duration of Nocturnal Hypoglycemia in Children Without Increasing Ketosis
Read this article at
Abstract
OBJECTIVE
Nocturnal hypoglycemia can cause seizures and is a major impediment to tight glycemic control, especially in young children with type 1 diabetes. We conducted an in-home randomized trial to assess the efficacy and safety of a continuous glucose monitor–based overnight predictive low-glucose suspend (PLGS) system.
RESEARCH DESIGN AND METHODS
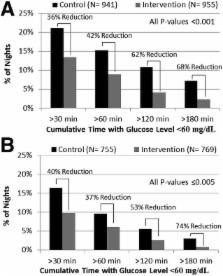
In two age-groups of children with type 1 diabetes (11–14 and 4–10 years of age), a 42-night trial for each child was conducted wherein each night was assigned randomly to either having the PLGS system active (intervention night) or inactive (control night). The primary outcome was percent time <70 mg/dL overnight.
RESULTS
Median time at <70 mg/dL was reduced by 54% from 10.1% on control nights to 4.6% on intervention nights ( P < 0.001) in 11–14-year-olds ( n = 45) and by 50% from 6.2% to 3.1% ( P < 0.001) in 4–10-year-olds ( n = 36). Mean overnight glucose was lower on control versus intervention nights in both age-groups (144 ± 18 vs. 152 ± 19 mg/dL [ P < 0.001] and 153 ± 14 vs. 160 ± 16 mg/dL [ P = 0.004], respectively). Mean morning blood glucose was 159 ± 29 vs. 176 ± 28 mg/dL ( P < 0.001) in the 11–14-year-olds and 154 ± 25 vs. 158 ± 22 mg/dL ( P = 0.11) in the 4–10-year-olds, respectively. No differences were found between intervention and control in either age-group in morning blood ketosis.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Outpatient glycemic control with a bionic pancreas in type 1 diabetes.
- Record: found
- Abstract: found
- Article: not found
Nocturnal glucose control with an artificial pancreas at a diabetes camp.
- Record: found
- Abstract: found
- Article: not found
