- Record: found
- Abstract: found
- Article: found
The role of cigarette smoke-induced epigenetic alterations in inflammation
Read this article at
Abstract
Background
Exposure to cigarette smoke (CS) is a major threat to human health worldwide. It is well established that smoking increases the risk of respiratory diseases, cardiovascular diseases and different forms of cancer, including lung, liver, and colon. CS-triggered inflammation is considered to play a central role in various pathologies by a mechanism that stimulates the release of pro-inflammatory cytokines. During this process, epigenetic alterations are known to play important roles in the specificity and duration of gene transcription.
Main text
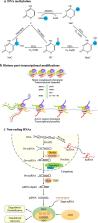
Epigenetic alterations include three major modifications: DNA modifications via methylation; various posttranslational modifications of histones, namely, methylation, acetylation, phosphorylation, and ubiquitination; and non-coding RNA sequences. These modifications work in concert to regulate gene transcription in a heritable fashion. The enzymes that regulate these epigenetic modifications can be activated by smoking, which further mediates the expression of multiple inflammatory genes. In this review, we summarize the current knowledge on the epigenetic alterations triggered by CS and assess how such alterations may affect smoking-mediated inflammatory responses.
Related collections
Most cited references173
- Record: found
- Abstract: found
- Article: not found
Novel role of PKR in inflammasome activation and HMGB1 release
- Record: found
- Abstract: found
- Article: not found
Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study.
- Record: found
- Abstract: found
- Article: found