- Record: found
- Abstract: found
- Article: found
High Affinity Neurexin Binding to Cell Adhesion G-protein-coupled Receptor CIRL1/Latrophilin-1 Produces an Intercellular Adhesion Complex*
Read this article at
Abstract
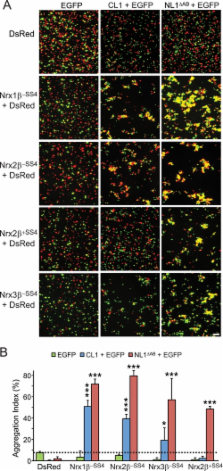
Background: Neurexins and CIRL/latrophilin-1 (CL1) are independent synaptic receptors for α-latrotoxin.
Results: Neurexins and CL1 form a high affinity complex that mediates intercellular adhesion and is regulated by neurexin alternative splicing.
Conclusion: Thus, two independent α-latrotoxin receptors interact trans-cellularly to form a connection between neurons.
Significance: The neurexin-CL1 complex may be involved in trans-synaptic cell adhesion and mediate α-latrotoxin toxicity.
Abstract
The G-protein-coupled receptor CIRL1/latrophilin-1 (CL1) and the type-1 membrane proteins neurexins represent distinct neuronal cell adhesion molecules that exhibit no similarities except for one common function: both proteins are receptors for α-latrotoxin, a component of black widow spider venom that induces massive neurotransmitter release at synapses. Unexpectedly, we have now identified a direct binding interaction between the extracellular domains of CL1 and neurexins that is regulated by alternative splicing of neurexins at splice site 4 (SS4). Using saturation binding assays, we showed that neurexins lacking an insert at SS4 bind to CL1 with nanomolar affinity, whereas neurexins containing an insert at SS4 are unable to bind. CL1 competed for neurexin binding with neuroligin-1, a well characterized neurexin ligand. The extracellular sequences of CL1 contain five domains (lectin, olfactomedin-like, serine/threonine-rich, hormone-binding, and G-protein-coupled receptor autoproteolysis-inducing (GAIN) domains). Of these domains, the olfactomedin-like domain mediates neurexin binding as shown by deletion mapping. Cell adhesion assays using cells expressing neurexins and CL1 revealed that their interaction produces a stable intercellular adhesion complex, indicating that their interaction can be trans-cellular. Thus, our data suggest that CL1 constitutes a novel ligand for neurexins that may be localized postsynaptically based on its well characterized interaction with intracellular SH3 and multiple ankyrin repeats adaptor proteins (SHANK) and could form a trans-synaptic complex with presynaptic neurexins.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons.
- Record: found
- Abstract: found
- Article: not found
Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins.
- Record: found
- Abstract: found
- Article: not found